
Contributions
Abstract: EP1139
Type: E-Poster Presentation
Session title: Platelet disorders
Background
Immune thrombocytopenia (ITP) is an acquired autoimmune disorder defined by a platelet count <100×109/L without explanation. This laboratory parameter has emerged as an important complication and have been shown to correlate with severe course in the coronavirus disease (COVID-19) caused by novel coronavirus SARS-CoV-2.
Aims
To analyze the clinical characteristics, diagnostic, blood count parameters, therapeutic strategies and outcomes in patients admitted with COVID-19 infection with acquired ITP.
Methods
An observational, longitudinal, descriptive and retrospective study was performed on all patients who were COVID-19 positive and platelet count < 100×109/L admitted to hospital between March 1 2020 and January 31 2021 at our centre. All patients were laboratory confirmed COVID-19 positive with a positive nasopharyngeal swab. Clinical and demographic data were collected for each patient, including age, gender, nadir platelet count, bleeding symptoms, time to recovery from start of treatment and clinical outcomes. Patients with previous hematological pathologies or thrombocytopenia were excluded. Mild COVID-19 disease was defined by presence of fever and cough only. Moderate disease was defined by presence of unilateral pneumonia by radiological evidence and/or clinical worsening. Severe disease was defined by presence of bilateral pneumonia by radiological evidence, admission to intensive care unit (ICU) during admission or met criteria for ARDS. Response to treatment was defined by documentation of platelet doubling or platelet count reaching at least > 30 × 109 /L (partial response-PR) or level reached > 100 × 109 /L or up to baseline count of that patient (complete response-CR). The results are expressed in percentages for qualitative variables, and in means and interquartilic ranges for continuous variables. The data were included in an Excel database (Microsoft®) and the IBM SPSS Statistics® 20 program was used for their analysis.
Results
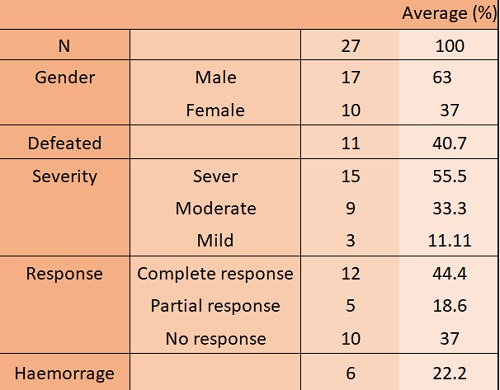
A total of 27 patients were identified, 17 males (62.96%) and 10 females (37.03%). The mean age at the time of the event was 78 years old (interquartile range-IQR-: 28 years). Attending to severity, 15 had severe disease (55.5%), 9 moderate and only 3 patients (11%) had a mild presentation.
The mean time from first COVID-19 manifestations to first ITP expression was 5 days (0–23). and in most of our patients was an incidental finding, presenting only 22% haemorragic clinic associated.
The majority of patients in our study did not receive a specific treatment for suspected ITP (e.g. only 6 were treated with intravenous immunoglobulins which were needed due to bleeding). But most patients were already treated with low doses of steroids as part of their treatment for COVID-19. The response rate was about 63% (PR and CR), with a mean of response of six days (IQR of 6.5 days).
Patients with severe disease and those who died at the end of the study, had lower platelet count than survivors and patients with moderate disease. However, our differences were not statisticaly significant.
Conclusion
In our study, ITP was more frequent in severe COVID-19, with lower nadir platelet count, as described in literature. Bleeding events were infrequent. Glucocorticoids were used as a treatment for COVID-19 disease, only used in few patients as ITP specific treatment. The results should be confirmed with prospective randomized controlled studies.
Keyword(s): COVID-19, Thrombocytopenia
Abstract: EP1139
Type: E-Poster Presentation
Session title: Platelet disorders
Background
Immune thrombocytopenia (ITP) is an acquired autoimmune disorder defined by a platelet count <100×109/L without explanation. This laboratory parameter has emerged as an important complication and have been shown to correlate with severe course in the coronavirus disease (COVID-19) caused by novel coronavirus SARS-CoV-2.
Aims
To analyze the clinical characteristics, diagnostic, blood count parameters, therapeutic strategies and outcomes in patients admitted with COVID-19 infection with acquired ITP.
Methods
An observational, longitudinal, descriptive and retrospective study was performed on all patients who were COVID-19 positive and platelet count < 100×109/L admitted to hospital between March 1 2020 and January 31 2021 at our centre. All patients were laboratory confirmed COVID-19 positive with a positive nasopharyngeal swab. Clinical and demographic data were collected for each patient, including age, gender, nadir platelet count, bleeding symptoms, time to recovery from start of treatment and clinical outcomes. Patients with previous hematological pathologies or thrombocytopenia were excluded. Mild COVID-19 disease was defined by presence of fever and cough only. Moderate disease was defined by presence of unilateral pneumonia by radiological evidence and/or clinical worsening. Severe disease was defined by presence of bilateral pneumonia by radiological evidence, admission to intensive care unit (ICU) during admission or met criteria for ARDS. Response to treatment was defined by documentation of platelet doubling or platelet count reaching at least > 30 × 109 /L (partial response-PR) or level reached > 100 × 109 /L or up to baseline count of that patient (complete response-CR). The results are expressed in percentages for qualitative variables, and in means and interquartilic ranges for continuous variables. The data were included in an Excel database (Microsoft®) and the IBM SPSS Statistics® 20 program was used for their analysis.
Results
A total of 27 patients were identified, 17 males (62.96%) and 10 females (37.03%). The mean age at the time of the event was 78 years old (interquartile range-IQR-: 28 years). Attending to severity, 15 had severe disease (55.5%), 9 moderate and only 3 patients (11%) had a mild presentation.
The mean time from first COVID-19 manifestations to first ITP expression was 5 days (0–23). and in most of our patients was an incidental finding, presenting only 22% haemorragic clinic associated.
The majority of patients in our study did not receive a specific treatment for suspected ITP (e.g. only 6 were treated with intravenous immunoglobulins which were needed due to bleeding). But most patients were already treated with low doses of steroids as part of their treatment for COVID-19. The response rate was about 63% (PR and CR), with a mean of response of six days (IQR of 6.5 days).
Patients with severe disease and those who died at the end of the study, had lower platelet count than survivors and patients with moderate disease. However, our differences were not statisticaly significant.

Conclusion
In our study, ITP was more frequent in severe COVID-19, with lower nadir platelet count, as described in literature. Bleeding events were infrequent. Glucocorticoids were used as a treatment for COVID-19 disease, only used in few patients as ITP specific treatment. The results should be confirmed with prospective randomized controlled studies.
Keyword(s): COVID-19, Thrombocytopenia


