Contributions
Abstract: EP1136
Type: E-Poster Presentation
Session title: Platelet disorders
Background
Acquired thrombotic thrombocytopenic purpura (aTTP) is caused by a deficiency in a von Willebrand factor (vWF) cleaving protease called ADAMTS13. Caplacizumab (C) is a monoclonal antibody that targets the A1 domain of vWF preventing platelet adhesion. The clinical trials that lead to the approval of C in aTTP have demonstrated improved outcomes. Experience outside clinical trials has been limited as many centers have never used this agent.
Aims
We report our single institution experience with C since it became commercially available. The aim of this study was to examine the efficacy and toxicity of C in patients with aTTP in standard practice.
Methods
This retrospective study included all patients admitted at our institution that were diagnosed with aTTP and received C since its approval. We used the following definitions through our data collection and analysis. Response: platelet count (PC) more than 150,000/uL and LDH less than 2 times the upper limit of normal (ULN). Exacerbation: aTTP event occurring within 30 days after end of plasma exchange (PLEX). Complete Response (CR): response without exacerbation. Relapse: aTTP event occurring more than 30 days after the end of PLEX. Day 1 was the first day of PLEX.
Results
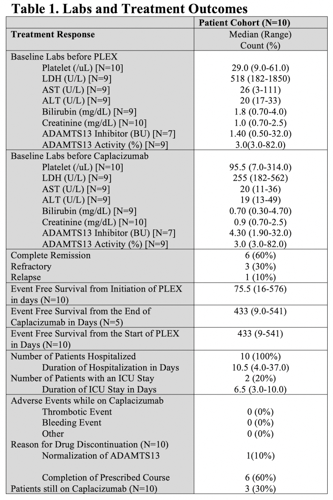
Patient characteristics: Ten patients with aTTP received C at our institution in 2019-2020. The median age of diagnosis was 52 years. Five (50%) patients were white, 3 (30%) were African American and 2 (20%) were Hispanic. Nobody had fever. Five (50%) subjects had neurologic symptoms (weakness, numbness, and altered mental status). Six (60%) patients had refractory aTTP while 4 (40%) had newly diagnosed aTTP at the time of starting C. Prior to receiving PLEX and C, 2 (20%) patients had bleeding events, one in the form of hematuria and another with severe anemia with a drop in hemoglobin of 2.0 g/dL in 24 hours requiring blood transfusion post thoracic aortic aneurysm repair. Two (20%) patients had a thrombotic stroke. Median (range) laboratory values prior to PLEX and C demonstrated: PC 29/uL (9-61), LDH 518 U/L (182-1850), ADAMTS13 activity 3.0% (3.0-82.0) and ADAMTS13 inhibitor 1.4 BU (0.5-32.0).
Treatments: All subjects received glucocorticoids, rituximab, PLEX and C. The median number of PLEX was 12 (1-33) days. C was started at a median of 5 (2-34) days after the start of PLEX and continued for median treatment duration of 33 (8-108) days with 3 patients still on C. Two patients received vincristine.
Efficacy: Time to normalized PC, LDH and ADAMTS13 activity in days were 5 (3-50), 3.5 (0-146), and 32.5 (0-89), respectively. The median event free survival from the start of PLEX was 75.5 (16-576) days. Six (60%) patients achieved CR, 3 (30%) had refractory disease and 1 (10%) had relapsed aTTP. No one had a major bleeding or major thrombotic event while taking C and no one died. The reason for C discontinuation was normalization of ADAMTS13 in 1 patient and completion of the prescribed course in the rest of our cohort.
Conclusion
Our study demonstrates that C is safe and effective at treating aTTP. C was associated with a short time to platelet normalization and number of PLEX days. No patient had a major bleeding event. While our population is small due to the rarity of aTTP, our data is important since it was derived from real-world experience and included all unselected patients. Finally, our data is consistent with clinical trial findings reported in the literature.
Keyword(s): ADAMTS13, Treatment, TTP
Abstract: EP1136
Type: E-Poster Presentation
Session title: Platelet disorders
Background
Acquired thrombotic thrombocytopenic purpura (aTTP) is caused by a deficiency in a von Willebrand factor (vWF) cleaving protease called ADAMTS13. Caplacizumab (C) is a monoclonal antibody that targets the A1 domain of vWF preventing platelet adhesion. The clinical trials that lead to the approval of C in aTTP have demonstrated improved outcomes. Experience outside clinical trials has been limited as many centers have never used this agent.
Aims
We report our single institution experience with C since it became commercially available. The aim of this study was to examine the efficacy and toxicity of C in patients with aTTP in standard practice.
Methods
This retrospective study included all patients admitted at our institution that were diagnosed with aTTP and received C since its approval. We used the following definitions through our data collection and analysis. Response: platelet count (PC) more than 150,000/uL and LDH less than 2 times the upper limit of normal (ULN). Exacerbation: aTTP event occurring within 30 days after end of plasma exchange (PLEX). Complete Response (CR): response without exacerbation. Relapse: aTTP event occurring more than 30 days after the end of PLEX. Day 1 was the first day of PLEX.
Results
Patient characteristics: Ten patients with aTTP received C at our institution in 2019-2020. The median age of diagnosis was 52 years. Five (50%) patients were white, 3 (30%) were African American and 2 (20%) were Hispanic. Nobody had fever. Five (50%) subjects had neurologic symptoms (weakness, numbness, and altered mental status). Six (60%) patients had refractory aTTP while 4 (40%) had newly diagnosed aTTP at the time of starting C. Prior to receiving PLEX and C, 2 (20%) patients had bleeding events, one in the form of hematuria and another with severe anemia with a drop in hemoglobin of 2.0 g/dL in 24 hours requiring blood transfusion post thoracic aortic aneurysm repair. Two (20%) patients had a thrombotic stroke. Median (range) laboratory values prior to PLEX and C demonstrated: PC 29/uL (9-61), LDH 518 U/L (182-1850), ADAMTS13 activity 3.0% (3.0-82.0) and ADAMTS13 inhibitor 1.4 BU (0.5-32.0).
Treatments: All subjects received glucocorticoids, rituximab, PLEX and C. The median number of PLEX was 12 (1-33) days. C was started at a median of 5 (2-34) days after the start of PLEX and continued for median treatment duration of 33 (8-108) days with 3 patients still on C. Two patients received vincristine.
Efficacy: Time to normalized PC, LDH and ADAMTS13 activity in days were 5 (3-50), 3.5 (0-146), and 32.5 (0-89), respectively. The median event free survival from the start of PLEX was 75.5 (16-576) days. Six (60%) patients achieved CR, 3 (30%) had refractory disease and 1 (10%) had relapsed aTTP. No one had a major bleeding or major thrombotic event while taking C and no one died. The reason for C discontinuation was normalization of ADAMTS13 in 1 patient and completion of the prescribed course in the rest of our cohort.
Conclusion
Our study demonstrates that C is safe and effective at treating aTTP. C was associated with a short time to platelet normalization and number of PLEX days. No patient had a major bleeding event. While our population is small due to the rarity of aTTP, our data is important since it was derived from real-world experience and included all unselected patients. Finally, our data is consistent with clinical trial findings reported in the literature.
Keyword(s): ADAMTS13, Treatment, TTP