
Contributions
Abstract: EP1134
Type: E-Poster Presentation
Session title: Platelet disorders
Background
Immune thrombocytopenia (ITP) is a rare immune-related condition characterized by low platelet counts and an increased haemorrhagic tendency. Romiplostim is a thrombopoietin receptor agonist (TPO-RA) used to treat ITP. Recent guidelines have recommended the earlier use of TPO-RAs following first-line therapy with corticosteroids, including in patients with ITP <1 year since diagnosis. However, outcomes of romiplostim treatment by ITP duration at initiation in real-world practice are required to strengthen the evidence in this setting.
Aims
We assessed effectiveness and safety of romiplostim within 24 weeks after initiation by duration of ITP: <3 (“newly diagnosed”), 3-12 (“persistent”), and >12 (“chronic”) months according to the updated product label. Real-world data collected between December 2010 and July 2017 in five Central and Eastern European countries were analysed.
Methods
This was a post-hoc analysis of the PLATON single arm, observational cohort study of adults receiving ≥1 dose of romiplostim within the indication approved at the time of study conduct (i.e. as second-line therapy after failure of other interventions, or where surgery was contraindicated). The planned observation period was 24 months. Durable platelet response was achieved if ≥75% of platelet count measurements during weeks 14-24 after romiplostim initiation were ≥50 x 109/L and no rescue therapy was administered. Overall platelet response was defined as at least one platelet measurement ≥50x109/L during weeks 2-24 after romiplostim initiation. Other outcomes included rescue therapy, bleeding, discontinuation of other ITP medications and adverse drug reactions (ADR). Patient and disease history were retrospectively collected at enrolment, prospective follow-up began at the first romiplostim dose and ended at the earliest of 24 months, loss to follow-up, censoring, or death.
Results
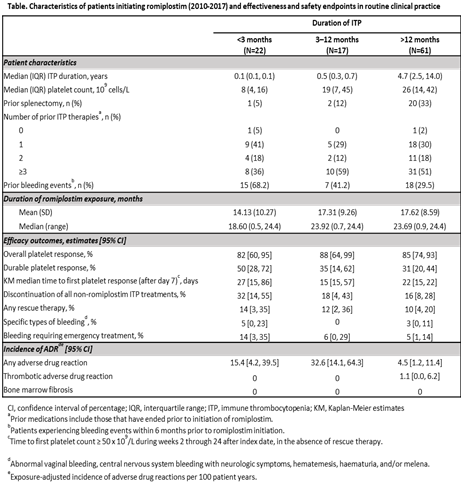
Of 100 patients, 22 (22%) were classified as newly diagnosed, 17 (17%) as persistent, and 61 (61%) as chronic at romiplostim initiation. The proportion of patients with splenectomy at initiation was highest in chronic patients (33%). Durable platelet response was achieved in 50% (CI: 28-72%) of newly diagnosed patients, 35% (CI: 14-62%) of patients with persistent ITP, and 31% (CI: 20-44%) of those with chronic ITP. Overall platelet response was achieved in >80% of patients across all strata. More newly diagnosed than persistent or chronic ITP patients discontinued all other concomitant ITP medications. Safety was comparable across ITP strata; the incidence of thrombotic ADRs was low and no occurrences of bone marrow ADRs were reported (Table).
Conclusion
In this real-world study in Central and Eastern Europe, platelet response to romiplostim was consistent between the early (newly diagnosed / persistent) and chronic ITP settings. ADRs were infrequent and similar across strata of ITP duration. These findings support the utilisation of romiplostim in patients with newly diagnosed and persistent ITP in accordance with recent guidelines and according to the romiplostim label extension for these patients.
Keyword(s): Bleeding, Immune thrombocytopenia (ITP), Thrombocytopenia, TPO
Abstract: EP1134
Type: E-Poster Presentation
Session title: Platelet disorders
Background
Immune thrombocytopenia (ITP) is a rare immune-related condition characterized by low platelet counts and an increased haemorrhagic tendency. Romiplostim is a thrombopoietin receptor agonist (TPO-RA) used to treat ITP. Recent guidelines have recommended the earlier use of TPO-RAs following first-line therapy with corticosteroids, including in patients with ITP <1 year since diagnosis. However, outcomes of romiplostim treatment by ITP duration at initiation in real-world practice are required to strengthen the evidence in this setting.
Aims
We assessed effectiveness and safety of romiplostim within 24 weeks after initiation by duration of ITP: <3 (“newly diagnosed”), 3-12 (“persistent”), and >12 (“chronic”) months according to the updated product label. Real-world data collected between December 2010 and July 2017 in five Central and Eastern European countries were analysed.
Methods
This was a post-hoc analysis of the PLATON single arm, observational cohort study of adults receiving ≥1 dose of romiplostim within the indication approved at the time of study conduct (i.e. as second-line therapy after failure of other interventions, or where surgery was contraindicated). The planned observation period was 24 months. Durable platelet response was achieved if ≥75% of platelet count measurements during weeks 14-24 after romiplostim initiation were ≥50 x 109/L and no rescue therapy was administered. Overall platelet response was defined as at least one platelet measurement ≥50x109/L during weeks 2-24 after romiplostim initiation. Other outcomes included rescue therapy, bleeding, discontinuation of other ITP medications and adverse drug reactions (ADR). Patient and disease history were retrospectively collected at enrolment, prospective follow-up began at the first romiplostim dose and ended at the earliest of 24 months, loss to follow-up, censoring, or death.
Results
Of 100 patients, 22 (22%) were classified as newly diagnosed, 17 (17%) as persistent, and 61 (61%) as chronic at romiplostim initiation. The proportion of patients with splenectomy at initiation was highest in chronic patients (33%). Durable platelet response was achieved in 50% (CI: 28-72%) of newly diagnosed patients, 35% (CI: 14-62%) of patients with persistent ITP, and 31% (CI: 20-44%) of those with chronic ITP. Overall platelet response was achieved in >80% of patients across all strata. More newly diagnosed than persistent or chronic ITP patients discontinued all other concomitant ITP medications. Safety was comparable across ITP strata; the incidence of thrombotic ADRs was low and no occurrences of bone marrow ADRs were reported (Table).

Conclusion
In this real-world study in Central and Eastern Europe, platelet response to romiplostim was consistent between the early (newly diagnosed / persistent) and chronic ITP settings. ADRs were infrequent and similar across strata of ITP duration. These findings support the utilisation of romiplostim in patients with newly diagnosed and persistent ITP in accordance with recent guidelines and according to the romiplostim label extension for these patients.
Keyword(s): Bleeding, Immune thrombocytopenia (ITP), Thrombocytopenia, TPO


