
Contributions
Abstract: EP1127
Type: E-Poster Presentation
Session title: Platelet disorders
Background
Immune Thrombocytopenic Purpura (ITP) is an immune mediated disorder characterized by an isolated decrease of the platelet count. Symptoms are usually mild, with cutaneous and/or mucosal bleeding, rarely serious symptoms occur, such as intracranial hemorrhage. ITP is classified into 3 groups: newly diagnosed (within 3 months from the onset), persistent (3 to 12 months from the onset), chronic (after 12 months from the onset). Infusion of immunoglobulin or steroid therapy are the first line of treatment. Current guidelines suggest the use of thrombopoietin receptor agonists (TPO-RAs), such as Eltrombopag (EPAG), in patients with persistent thrombocytopenia. They stimulate thrombopoiesis through the activation of the thrombopoietin receptors, furthermore TPO-RAs can influence platelet antibody production and T regulatory cell count. EPAG is an effective and safe therapy, although its use is currently approved in patients with ITP after at least 6 months from the diagnosis. Some studies, in the adult setting, have already described the safety and efficacy of EPAG in patients before the 6th month from the onset (off label use).Currently there are no studies on the use of EPAG in the same setting in a cohort of pediatric patients with ITP.
Aims
Evaluate the safety and efficacy of the use of EPAG within 6 months from the onset on a cohort of pediatric patients with ITP.
Methods
Study population: 59 patients aged between 1 and 18 years were enrolled from February 2013 and December 2020.
Response evaluation: We evaluated the type of response based on the platelet count: complete if the platelet count was at least > 100.000 in one determination, partial if the platelet count was at least once between 30.000 and 100.000, absent if the platelet count never overcame 30.000.
We also defined the persistent response as a platelet count constantly over 30.000, in the absence of symptoms nor rescue therapies over a follow-up of at least 1 year.
Results
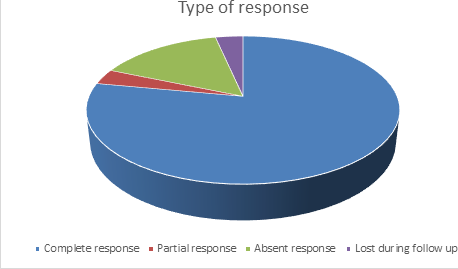
We enrolled 59 patients, 39 with newly diagnosed ITP and 20 with persistent ITP. 46 patients had a complete response (80,7%), 2 patients (3,5%) had a partial response, 9 patients did not respond to EPAG. 2 patients were lost at follow-up. 39 of the 49 patients who responded to the treatment with EPAG had a follow-up longer than 1 year, allowing the evaluation of the persistence of the response. 29 patients (74,4%) had a persistent response, with no differences between the group with newly diagnosed ITP and persistent ITP. 14 patients (23,7%) had at least one adverse event. 3 patients presented with significant thrombocytosis (over 1.000.000) that promptly resolved after a temporary, brief suspension of the treatment. Other adverse events were hypertransaminasemia, nausea, hypoferritinemia with or without anemia. In one patient who had grade 3 hypertransaminasemia and acydosis EPAG was suspended.
Conclusion
This study is, to the best of our knowledge, the first reporting on the use of EPAG in a pediatric population with newly diagnosed or persistent (3-6 months) ITP. Our data highlight the efficacy of this therapy and its safety, with only a small proportion of patients reporting reversible adverse events. Therefore, given the difficulties to treat patients who do not respond to the first line therapy or who have an early relapse, EPAG could be an option.
We believe further studies are needed to explore the use of EPAG in these contexts.
Keyword(s): Immune thrombocytopenia (ITP), Pediatric, Thrombopoietin (TPO)
Abstract: EP1127
Type: E-Poster Presentation
Session title: Platelet disorders
Background
Immune Thrombocytopenic Purpura (ITP) is an immune mediated disorder characterized by an isolated decrease of the platelet count. Symptoms are usually mild, with cutaneous and/or mucosal bleeding, rarely serious symptoms occur, such as intracranial hemorrhage. ITP is classified into 3 groups: newly diagnosed (within 3 months from the onset), persistent (3 to 12 months from the onset), chronic (after 12 months from the onset). Infusion of immunoglobulin or steroid therapy are the first line of treatment. Current guidelines suggest the use of thrombopoietin receptor agonists (TPO-RAs), such as Eltrombopag (EPAG), in patients with persistent thrombocytopenia. They stimulate thrombopoiesis through the activation of the thrombopoietin receptors, furthermore TPO-RAs can influence platelet antibody production and T regulatory cell count. EPAG is an effective and safe therapy, although its use is currently approved in patients with ITP after at least 6 months from the diagnosis. Some studies, in the adult setting, have already described the safety and efficacy of EPAG in patients before the 6th month from the onset (off label use).Currently there are no studies on the use of EPAG in the same setting in a cohort of pediatric patients with ITP.
Aims
Evaluate the safety and efficacy of the use of EPAG within 6 months from the onset on a cohort of pediatric patients with ITP.
Methods
Study population: 59 patients aged between 1 and 18 years were enrolled from February 2013 and December 2020.
Response evaluation: We evaluated the type of response based on the platelet count: complete if the platelet count was at least > 100.000 in one determination, partial if the platelet count was at least once between 30.000 and 100.000, absent if the platelet count never overcame 30.000.
We also defined the persistent response as a platelet count constantly over 30.000, in the absence of symptoms nor rescue therapies over a follow-up of at least 1 year.
Results
We enrolled 59 patients, 39 with newly diagnosed ITP and 20 with persistent ITP. 46 patients had a complete response (80,7%), 2 patients (3,5%) had a partial response, 9 patients did not respond to EPAG. 2 patients were lost at follow-up. 39 of the 49 patients who responded to the treatment with EPAG had a follow-up longer than 1 year, allowing the evaluation of the persistence of the response. 29 patients (74,4%) had a persistent response, with no differences between the group with newly diagnosed ITP and persistent ITP. 14 patients (23,7%) had at least one adverse event. 3 patients presented with significant thrombocytosis (over 1.000.000) that promptly resolved after a temporary, brief suspension of the treatment. Other adverse events were hypertransaminasemia, nausea, hypoferritinemia with or without anemia. In one patient who had grade 3 hypertransaminasemia and acydosis EPAG was suspended.

Conclusion
This study is, to the best of our knowledge, the first reporting on the use of EPAG in a pediatric population with newly diagnosed or persistent (3-6 months) ITP. Our data highlight the efficacy of this therapy and its safety, with only a small proportion of patients reporting reversible adverse events. Therefore, given the difficulties to treat patients who do not respond to the first line therapy or who have an early relapse, EPAG could be an option.
We believe further studies are needed to explore the use of EPAG in these contexts.
Keyword(s): Immune thrombocytopenia (ITP), Pediatric, Thrombopoietin (TPO)


