
Contributions
Abstract: EP1124
Type: E-Poster Presentation
Session title: Platelet disorders
Background
Primary Immune Thrombocytopenia (ITP) is an autoimmune disorder, characterized by low platelets counts due to immune-mediated platelet destruction. The spleen plays a central role in the pathogenesis of ITP, as it is the main site of anti-platelet immune responses and platelet destruction. Thrombopoietin receptor agonists (TPO-RA) are a valid second-line therapy for ITP, being associated with increased platelet production and immune modulatory effects. Such immunological mechanisms are poorly characterized and nothing is known on the splenic effects of TPO-RA administration.
Aims
This study aimed at assessing: (i) the immunological effects of TPO-RA on ITP spleens; and (ii) the effects of TPO-RA administration on splenectomy outcome.
Methods
A clinically annotated series of 35 ITP spleens removed from therapeutic purposes was histologically assessed for the following parameters: (i) white pulp features (lymphoid follicle density and marginal zone width); (iii) subset of CD207/PD1 positive T-cells(which include T follicular helper (Tfh) cells; (iv) cytotoxic T cell density (as assessed by CD8 immunostain); (v) Th1 cell density (as assessed by tBet immunostain); (vi) Th2 cell density (as assessed by GATA3 immunostain); (vii) Treg cell density (as assessed by FOXP3 immunostain). Ten non-ITP spleens were used as controls. ITP patients were stratified into responders and non-responders to splenectomy, based on IWG consensus criteria. Statistical analysis was performed by non-parametric tests for quantitative (Mann-Whitney-Wilcoxon test) and qualitative (Fisher’s exact test). Kaplan-Meyer and log rank methods were used for survival analyses. Differences between groups were considered statistically significant for p-values <0.05.
Results
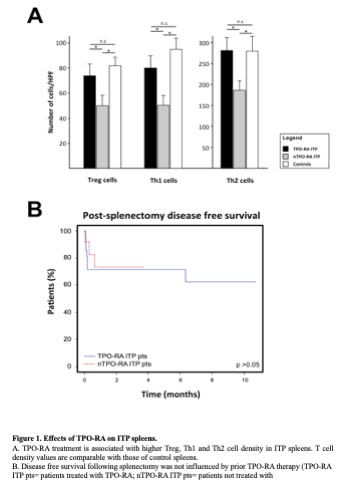
The study population included 12/35 (34.3%) males and 23/35 (65.7%) females (M:F ratio: 0.5) with mean age at splenectomy of 41.0 years (range: 16.8 – 68.2 years). TPO-RAs were administered in 13/35 (37.1%) cases. The spleens of TPO-RA treated patients had higher Treg, Th1 and Th2 densities compared to TPO-RA untreated patients (Treg density: 73.7 cells/HPF vs 49.4 cells; Th1 density: 79.8 cells/HPF vs 49.8 cells/HPF; Th2 density: 281.6 cells/HPF vs 185.9 cells/HPF; p< 0.05; Figure 1A). No other differences were noted between TPO-RA treated and untreated cases. Splenic Treg, Th1 and Th2 densities of TPO-RA treated ITP were similar to those of non-ITP controls (p> 0.05). In ITP patients, the outcome of splenectomy was not influenced by prior TPO-RA treatment and/or splenic T cell patterns (Figure 1B).
Conclusion
Treatment with TPO-RAs in ITP is associated with increased splenic Treg, Th1 and Th2 cells, at values comparable to those of healthy controls. TPO-RA does not influence the outcome of splenectomy in primary ITP.
Keyword(s): Immune thrombocytopenia (ITP), Splenectomy, Thrombocytopenia
Abstract: EP1124
Type: E-Poster Presentation
Session title: Platelet disorders
Background
Primary Immune Thrombocytopenia (ITP) is an autoimmune disorder, characterized by low platelets counts due to immune-mediated platelet destruction. The spleen plays a central role in the pathogenesis of ITP, as it is the main site of anti-platelet immune responses and platelet destruction. Thrombopoietin receptor agonists (TPO-RA) are a valid second-line therapy for ITP, being associated with increased platelet production and immune modulatory effects. Such immunological mechanisms are poorly characterized and nothing is known on the splenic effects of TPO-RA administration.
Aims
This study aimed at assessing: (i) the immunological effects of TPO-RA on ITP spleens; and (ii) the effects of TPO-RA administration on splenectomy outcome.
Methods
A clinically annotated series of 35 ITP spleens removed from therapeutic purposes was histologically assessed for the following parameters: (i) white pulp features (lymphoid follicle density and marginal zone width); (iii) subset of CD207/PD1 positive T-cells(which include T follicular helper (Tfh) cells; (iv) cytotoxic T cell density (as assessed by CD8 immunostain); (v) Th1 cell density (as assessed by tBet immunostain); (vi) Th2 cell density (as assessed by GATA3 immunostain); (vii) Treg cell density (as assessed by FOXP3 immunostain). Ten non-ITP spleens were used as controls. ITP patients were stratified into responders and non-responders to splenectomy, based on IWG consensus criteria. Statistical analysis was performed by non-parametric tests for quantitative (Mann-Whitney-Wilcoxon test) and qualitative (Fisher’s exact test). Kaplan-Meyer and log rank methods were used for survival analyses. Differences between groups were considered statistically significant for p-values <0.05.
Results
The study population included 12/35 (34.3%) males and 23/35 (65.7%) females (M:F ratio: 0.5) with mean age at splenectomy of 41.0 years (range: 16.8 – 68.2 years). TPO-RAs were administered in 13/35 (37.1%) cases. The spleens of TPO-RA treated patients had higher Treg, Th1 and Th2 densities compared to TPO-RA untreated patients (Treg density: 73.7 cells/HPF vs 49.4 cells; Th1 density: 79.8 cells/HPF vs 49.8 cells/HPF; Th2 density: 281.6 cells/HPF vs 185.9 cells/HPF; p< 0.05; Figure 1A). No other differences were noted between TPO-RA treated and untreated cases. Splenic Treg, Th1 and Th2 densities of TPO-RA treated ITP were similar to those of non-ITP controls (p> 0.05). In ITP patients, the outcome of splenectomy was not influenced by prior TPO-RA treatment and/or splenic T cell patterns (Figure 1B).

Conclusion
Treatment with TPO-RAs in ITP is associated with increased splenic Treg, Th1 and Th2 cells, at values comparable to those of healthy controls. TPO-RA does not influence the outcome of splenectomy in primary ITP.
Keyword(s): Immune thrombocytopenia (ITP), Splenectomy, Thrombocytopenia


