
Contributions
Abstract: EP1119
Type: E-Poster Presentation
Session title: Myeloproliferative neoplasms - Clinical
Background
Despite significant benefits of ruxolitinib (RUX) in MPN management, e.g. primary (PMF) or secondary myelofibrosis and polycythemia vera (PV), the reports of opportunistic infections occurring during RUX treatment, particularly pulmonary (PTB) or disseminated tuberculosis (DTB), are increasing worldwide.
Aims
To identify PTB/DTB cases in RUX-treated MPN and report on epidemiology, clinical manifestations, RUX associations, outcomes.
Methods
Systematic search computed in PubMed/Medline, Web of Science, ScienceDirect, SCOPUS, from the inception of these databases until 15.11.2020. Relevant English-written articles were identified based on the Eligibility criteria: 1. Confirmed MPN diagnosis, 2. Confirmed diagnosis of PTB/DTB caused by Mycobacterium tuberculosis, 3. MPN managed with RUX. Exclusion criteria: reports of other mycobacteria apart from M. tuberculosis, RUX used in other disorders, in vitro studies, animal studies. The initial search identified 156 publications, of which 22 were selected based on title/abstract screening. Following full-text review, 20 publications depicting 26 distinct case reports were included in the systematic review and meta-analysis.
Results
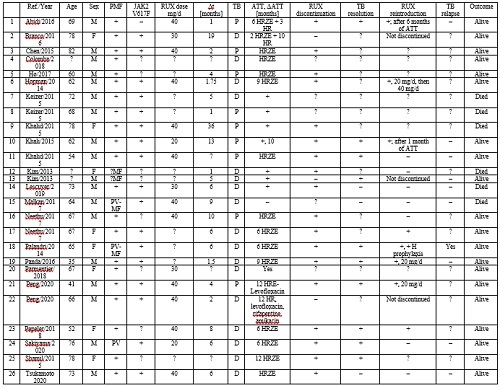
26 case reports were identified: 69.2% females, mean age 65.6 years, 80.8% PMF. JAK2V617F mutation was detected in 65.4% of cases. RUX mean dose was 35 mg/day (40.74% received 40 mg/day) and the average time from RUX initiation to TB diagnosis was 7 months. TB screening was performed only in 19.2% of subjects. DTB was more frequent versus PTB (65.4% versus 34.6%). PTB and DTP were observed primarily in PMF (80.8%) and less frequently in post-PV-MF (7.7%) or PV (3.85%). The most common presentations were fever (80.7%), peripheral lymphadenopathy (38.5%), night sweats (23.1%), fatigue/tiredness (19.2%), weight loss (15.4%), cough (11.54%) and shortness of breath (7.7%). All patients received 6, 9 or 12 months of antitubercular treatment (ATT), i.e. HRZE (isoniazid + rifampicin + pyrazinamide + ethambutol) or variants. RUX was discontinued in 69.2% of cases and maintained in 11.5% of cases in combination with ATT. RUX re-initiation was decided in 23.1% of patients. TB-related death occurred in 23.1% of the subjects involved. Most case reports did not report on follow-up visits, thus assessing TB resolution or relapse is difficult to pursue. The attached table details the case reports of RUX-associated TB included in our analysis (+ = Yes; – = No; ? = Unknown/Unspecified/Unavailable; P-pulmonary, D-disseminated TB).
Conclusion
Opportunistic infections, including PTB/DTB, can develop during RUX treatment due to its immunosuppressive effects. Screening for TB is advised prior to RUX prescription by skin tests, IFN-γ releasing assays and (or) chest X-rays. Prophylaxis can be considered in TB-endemic areas.
Keyword(s): Myelofibrosis, Ruxolitinib, Screening, Tuberculosis
Abstract: EP1119
Type: E-Poster Presentation
Session title: Myeloproliferative neoplasms - Clinical
Background
Despite significant benefits of ruxolitinib (RUX) in MPN management, e.g. primary (PMF) or secondary myelofibrosis and polycythemia vera (PV), the reports of opportunistic infections occurring during RUX treatment, particularly pulmonary (PTB) or disseminated tuberculosis (DTB), are increasing worldwide.
Aims
To identify PTB/DTB cases in RUX-treated MPN and report on epidemiology, clinical manifestations, RUX associations, outcomes.
Methods
Systematic search computed in PubMed/Medline, Web of Science, ScienceDirect, SCOPUS, from the inception of these databases until 15.11.2020. Relevant English-written articles were identified based on the Eligibility criteria: 1. Confirmed MPN diagnosis, 2. Confirmed diagnosis of PTB/DTB caused by Mycobacterium tuberculosis, 3. MPN managed with RUX. Exclusion criteria: reports of other mycobacteria apart from M. tuberculosis, RUX used in other disorders, in vitro studies, animal studies. The initial search identified 156 publications, of which 22 were selected based on title/abstract screening. Following full-text review, 20 publications depicting 26 distinct case reports were included in the systematic review and meta-analysis.
Results
26 case reports were identified: 69.2% females, mean age 65.6 years, 80.8% PMF. JAK2V617F mutation was detected in 65.4% of cases. RUX mean dose was 35 mg/day (40.74% received 40 mg/day) and the average time from RUX initiation to TB diagnosis was 7 months. TB screening was performed only in 19.2% of subjects. DTB was more frequent versus PTB (65.4% versus 34.6%). PTB and DTP were observed primarily in PMF (80.8%) and less frequently in post-PV-MF (7.7%) or PV (3.85%). The most common presentations were fever (80.7%), peripheral lymphadenopathy (38.5%), night sweats (23.1%), fatigue/tiredness (19.2%), weight loss (15.4%), cough (11.54%) and shortness of breath (7.7%). All patients received 6, 9 or 12 months of antitubercular treatment (ATT), i.e. HRZE (isoniazid + rifampicin + pyrazinamide + ethambutol) or variants. RUX was discontinued in 69.2% of cases and maintained in 11.5% of cases in combination with ATT. RUX re-initiation was decided in 23.1% of patients. TB-related death occurred in 23.1% of the subjects involved. Most case reports did not report on follow-up visits, thus assessing TB resolution or relapse is difficult to pursue. The attached table details the case reports of RUX-associated TB included in our analysis (+ = Yes; – = No; ? = Unknown/Unspecified/Unavailable; P-pulmonary, D-disseminated TB).

Conclusion
Opportunistic infections, including PTB/DTB, can develop during RUX treatment due to its immunosuppressive effects. Screening for TB is advised prior to RUX prescription by skin tests, IFN-γ releasing assays and (or) chest X-rays. Prophylaxis can be considered in TB-endemic areas.
Keyword(s): Myelofibrosis, Ruxolitinib, Screening, Tuberculosis


