
Contributions
Abstract: EP1109
Type: E-Poster Presentation
Session title: Myeloproliferative neoplasms - Clinical
Background
Erythrocytosis is a condition of increased red cell mass, manifested by high haemoglobin (Hb) and/or haematocrit (Hct). Most frequently, it is secondary (SE) to hypoxia caused by various cardiac, pulmonary or renal diseases. Congenital causes are very rare. The only clonal erythrocytosis is polycythaemia vera (PV), almost uniformly associated with JAK2 mutation. When no cause of erythrocytosis is identified, the erythrocytosis is idiopathic (IE). As thromboembolic events are frequent in PV, patients are usually treated with antiaggregation and cytoreduction. Management of all other types of erythrocytosis is less clear and differs according to the underlying cause of erythrocytosis
Aims
The aim of our study was to retrospectively analyze the etiology and management of patients with non-clonal erythrocytosis referred to an adult haematology outpatient clinic in an 8-year period.
Methods
First step was inclusion of patients with Hb> 185 g/L and/or Hct> 0.52 in men and Hb> 165 g/L and/or Hct> 0.48 in women on two visits ≥ two months apart, thus confirming absolute erythrocytosis. Secondly, PV was excluded by determination of JAK2 mutation and erythropoietin level, sometimes also by bone marrow biopsy. Concomitantly, causes of SE were identified using various biochemistry and imaging techniques (iron stores, haemochromatosis testing, pulse oximetry, blood gas analysis with COHb determination, CT or ultrasound for detection of cardiac or pulmonary disease or origin of ectopic erythropoietin, polysomnography). Thirdly, IE patients were referred to targeted next-generation sequencing for congenital erythrocytosis. Informed consent was obtained from all participants.
Results
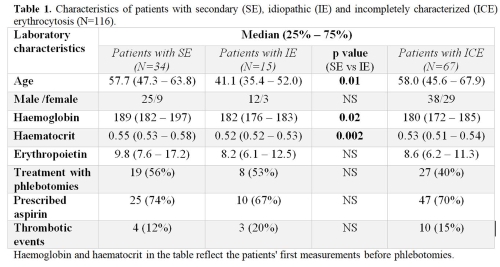
Between March 2011 and April 2019, 655 patients met the inclusion criteria for absolute erythrocytosis. After excluding PV 116 patients were evaluated for other causes of erythrocytosis; 75 (65%) were men and 41 (35%) women. Median age in men and women was 51.4 years (41.1 – 62.9, interquartile range), and 60.7 years (54.4 -68.2), respectively (p = 0.002). For 34/116 (29%) patients secondary causes of erythrocytosis were identified, namely obstructive sleep apnoea (38%), smoking (21%), lung (18%) and cardiovascular diseases (6%), neurological disorders affecting respiratory function (6%), kidney diseases (renal cell carcinoma, renal cysts, polycystic kidney disease) (12%), tumors with ectopic erythropoietin secretion (6%), hemochromatosis (3%) and drugs (6%). 15/116 (13%) patients remained idiopathic despite extensive diagnostics. 67/116 (58%) patients stayed incompletely characterized (ICE) due to insufficient diagnostics. Patients from all three groups were similarly treated, either with aspirin or phlebotomies. Differences in SE, IE and ICE patients are presented in Table 1. Thrombotic events were registered in 12%, 20% and 15% of SE, IE and ICE patients, respectively. Congenital erythrocytosis type 4 (ECYT4) was diagnosed in one patient, who had already suffered several thromboembolic complications.
Conclusion
Our study demonstrated that diagnostics of erythrocytosis often stopped after PV exclusion. Thromboembolic events were not rare among patients with non-clonal erythrocytosis, which could be attributable to yet unidentified causes of congenital erythrocytosis in the ICE group but also to inadequate management of secondary causes. The optimal management for each patient can be chosen only by identifying the etiology of erythrocytosis.
Keyword(s): Erythrocytosis
Abstract: EP1109
Type: E-Poster Presentation
Session title: Myeloproliferative neoplasms - Clinical
Background
Erythrocytosis is a condition of increased red cell mass, manifested by high haemoglobin (Hb) and/or haematocrit (Hct). Most frequently, it is secondary (SE) to hypoxia caused by various cardiac, pulmonary or renal diseases. Congenital causes are very rare. The only clonal erythrocytosis is polycythaemia vera (PV), almost uniformly associated with JAK2 mutation. When no cause of erythrocytosis is identified, the erythrocytosis is idiopathic (IE). As thromboembolic events are frequent in PV, patients are usually treated with antiaggregation and cytoreduction. Management of all other types of erythrocytosis is less clear and differs according to the underlying cause of erythrocytosis
Aims
The aim of our study was to retrospectively analyze the etiology and management of patients with non-clonal erythrocytosis referred to an adult haematology outpatient clinic in an 8-year period.
Methods
First step was inclusion of patients with Hb> 185 g/L and/or Hct> 0.52 in men and Hb> 165 g/L and/or Hct> 0.48 in women on two visits ≥ two months apart, thus confirming absolute erythrocytosis. Secondly, PV was excluded by determination of JAK2 mutation and erythropoietin level, sometimes also by bone marrow biopsy. Concomitantly, causes of SE were identified using various biochemistry and imaging techniques (iron stores, haemochromatosis testing, pulse oximetry, blood gas analysis with COHb determination, CT or ultrasound for detection of cardiac or pulmonary disease or origin of ectopic erythropoietin, polysomnography). Thirdly, IE patients were referred to targeted next-generation sequencing for congenital erythrocytosis. Informed consent was obtained from all participants.
Results
Between March 2011 and April 2019, 655 patients met the inclusion criteria for absolute erythrocytosis. After excluding PV 116 patients were evaluated for other causes of erythrocytosis; 75 (65%) were men and 41 (35%) women. Median age in men and women was 51.4 years (41.1 – 62.9, interquartile range), and 60.7 years (54.4 -68.2), respectively (p = 0.002). For 34/116 (29%) patients secondary causes of erythrocytosis were identified, namely obstructive sleep apnoea (38%), smoking (21%), lung (18%) and cardiovascular diseases (6%), neurological disorders affecting respiratory function (6%), kidney diseases (renal cell carcinoma, renal cysts, polycystic kidney disease) (12%), tumors with ectopic erythropoietin secretion (6%), hemochromatosis (3%) and drugs (6%). 15/116 (13%) patients remained idiopathic despite extensive diagnostics. 67/116 (58%) patients stayed incompletely characterized (ICE) due to insufficient diagnostics. Patients from all three groups were similarly treated, either with aspirin or phlebotomies. Differences in SE, IE and ICE patients are presented in Table 1. Thrombotic events were registered in 12%, 20% and 15% of SE, IE and ICE patients, respectively. Congenital erythrocytosis type 4 (ECYT4) was diagnosed in one patient, who had already suffered several thromboembolic complications.

Conclusion
Our study demonstrated that diagnostics of erythrocytosis often stopped after PV exclusion. Thromboembolic events were not rare among patients with non-clonal erythrocytosis, which could be attributable to yet unidentified causes of congenital erythrocytosis in the ICE group but also to inadequate management of secondary causes. The optimal management for each patient can be chosen only by identifying the etiology of erythrocytosis.
Keyword(s): Erythrocytosis


