
Contributions
Abstract: EP1106
Type: E-Poster Presentation
Session title: Myeloproliferative neoplasms - Clinical
Background
Imetelstat is a first-in-class telomerase inhibitor that induces selective apoptosis of malignant hematopoietic stem and progenitor cells in the bone marrow. Imetelstat has shown clinical benefit in symptom response rate and a potential overall survival (OS) impact in Janus kinase (JAK) inhibitor relapsed and refractory (R/R) intermediate 2 (Int-2) and high risk (HR) myelofibrosis (MF) patients (pts) in the Phase 2 MYF2001 (IMbark) study, as well as meaningful and durable transfusion independence (TI) in high transfusion burden low risk (LR) and intermediate 1 (Int-1) risk myelodysplastic syndrome (MDS) pts R/R to erythropoiesis stimulating agents (ESAs) in the Phase 2 portion of the MDS3001 (IMerge) study. Clinical and biomarker data in both studies indicate potential disease-modifying activity of imetelstat. The most frequently reported adverse events (AEs) in both studies were manageable and reversible cytopenias, with limited clinical consequences, suggesting on-target activity of imetelstat (Platzbecker ASH 2020, Mascarenhas ASH 2020).
Aims
To further characterize hematologic and non-hematologic AEs, including the AEs of Interest (AEI), hepatic AEs and grade ≥3 liver function tests (LFTs) in these Phase 2 studies.
Methods
These safety analyses focus on MYF2001 study pts randomized to imetelsat 9.4 mg/kg IV q 3 weeks (wks) (N=59) as of 7 Feb 2020 and the Phase 2 of the MDS3001 study in LR/Int-1 non-del(5q) transfusion dependent ESA R/R MDS pts, all lenalidomide and hypomethylating agent-naïve (N=38), who received 7.5 mg/kg IV every 4 wks as of 21 Oct 2020. AEs were monitored and reported from the time of screening until 30 days after treatment discontinuation. Dose modification was performed based on protocol-defined criteria. An independent hepatic expert committee (HEC) reviewed all LFT and AEI data quarterly.
Results
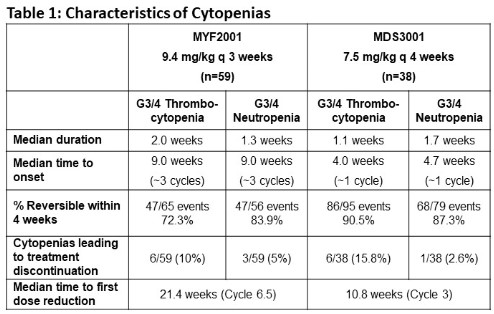
On MYF2001 41% and 32% of pts had grade (G) ≥3 (G≥3) thrombocytopenia and neutropenia respectively and on MDS3001 61% and 55% of pts had G≥3 thrombocytopenia and neutropenia respectively. Median time to onset of these G≥ 3 cytopenias was 3 cycles in MF pts and approximately 1 cycle in MDS pts. Median duration of G≥3 cytopenias was <2 wks and >72% were reversible within 4 wks (Table 1). Median time to first dose reduction was 21 wks in MF and 11 wks in MDS. Cytopenias leading to treatment discontinuation occurred in 15% and 18% of MF and MDS pts, respectively. Importantly, clinical consequences of cytopenias including G≥3 hemorrhagic events or febrile neutropenia occurred in <10% of pts. Non-hematological AEs occurring in >20% pts in either study were nausea, diarrhea, abdominal pain, fatigue, asthenia, pyrexia, dyspnea, and back pain and were generally G1-2 events. One pt on each study experienced imetelstat related G3 AST increase that recovered with dose reduction. HEC reviews found no significant imetelstat-related liver injury.
Conclusion
Imetelstat related cytopenias are on-target effects based on the selective reduction of malignant cells through telomerase inhibition. Furthermore, they are of short duration, reversible and with limited clinical consequences when managed with the dose modification guidelines in the protocols. Pts in these two studies had no evidence of imetelstat-related liver injury. Differences in disease pathology (proliferation vs dysplasia) could account for the differences in toxicity profiles in MF vs MDS pts. Ongoing Phase 3 studies in MF and MDS will confirm the safety profile of imetelstat in a controlled setting.
Keyword(s): Myelodysplasia, Myelofibrosis, Safety, Telomerase
Abstract: EP1106
Type: E-Poster Presentation
Session title: Myeloproliferative neoplasms - Clinical
Background
Imetelstat is a first-in-class telomerase inhibitor that induces selective apoptosis of malignant hematopoietic stem and progenitor cells in the bone marrow. Imetelstat has shown clinical benefit in symptom response rate and a potential overall survival (OS) impact in Janus kinase (JAK) inhibitor relapsed and refractory (R/R) intermediate 2 (Int-2) and high risk (HR) myelofibrosis (MF) patients (pts) in the Phase 2 MYF2001 (IMbark) study, as well as meaningful and durable transfusion independence (TI) in high transfusion burden low risk (LR) and intermediate 1 (Int-1) risk myelodysplastic syndrome (MDS) pts R/R to erythropoiesis stimulating agents (ESAs) in the Phase 2 portion of the MDS3001 (IMerge) study. Clinical and biomarker data in both studies indicate potential disease-modifying activity of imetelstat. The most frequently reported adverse events (AEs) in both studies were manageable and reversible cytopenias, with limited clinical consequences, suggesting on-target activity of imetelstat (Platzbecker ASH 2020, Mascarenhas ASH 2020).
Aims
To further characterize hematologic and non-hematologic AEs, including the AEs of Interest (AEI), hepatic AEs and grade ≥3 liver function tests (LFTs) in these Phase 2 studies.
Methods
These safety analyses focus on MYF2001 study pts randomized to imetelsat 9.4 mg/kg IV q 3 weeks (wks) (N=59) as of 7 Feb 2020 and the Phase 2 of the MDS3001 study in LR/Int-1 non-del(5q) transfusion dependent ESA R/R MDS pts, all lenalidomide and hypomethylating agent-naïve (N=38), who received 7.5 mg/kg IV every 4 wks as of 21 Oct 2020. AEs were monitored and reported from the time of screening until 30 days after treatment discontinuation. Dose modification was performed based on protocol-defined criteria. An independent hepatic expert committee (HEC) reviewed all LFT and AEI data quarterly.
Results
On MYF2001 41% and 32% of pts had grade (G) ≥3 (G≥3) thrombocytopenia and neutropenia respectively and on MDS3001 61% and 55% of pts had G≥3 thrombocytopenia and neutropenia respectively. Median time to onset of these G≥ 3 cytopenias was 3 cycles in MF pts and approximately 1 cycle in MDS pts. Median duration of G≥3 cytopenias was <2 wks and >72% were reversible within 4 wks (Table 1). Median time to first dose reduction was 21 wks in MF and 11 wks in MDS. Cytopenias leading to treatment discontinuation occurred in 15% and 18% of MF and MDS pts, respectively. Importantly, clinical consequences of cytopenias including G≥3 hemorrhagic events or febrile neutropenia occurred in <10% of pts. Non-hematological AEs occurring in >20% pts in either study were nausea, diarrhea, abdominal pain, fatigue, asthenia, pyrexia, dyspnea, and back pain and were generally G1-2 events. One pt on each study experienced imetelstat related G3 AST increase that recovered with dose reduction. HEC reviews found no significant imetelstat-related liver injury.

Conclusion
Imetelstat related cytopenias are on-target effects based on the selective reduction of malignant cells through telomerase inhibition. Furthermore, they are of short duration, reversible and with limited clinical consequences when managed with the dose modification guidelines in the protocols. Pts in these two studies had no evidence of imetelstat-related liver injury. Differences in disease pathology (proliferation vs dysplasia) could account for the differences in toxicity profiles in MF vs MDS pts. Ongoing Phase 3 studies in MF and MDS will confirm the safety profile of imetelstat in a controlled setting.
Keyword(s): Myelodysplasia, Myelofibrosis, Safety, Telomerase


