
Contributions
Abstract: EP1095
Type: E-Poster Presentation
Session title: Myeloproliferative neoplasms - Clinical
Background
Mastocytosis is a heterogeneous group of rare diseases related to the proliferation and/or abnormal accumulation of mast cells in one or more organs. The most common form, observed mostly in children, is cutaneous mastocytosis where the infiltration of mast cells is limited to the skin. When it reaches other organs, the disease is called systemic mastocytosis (SM). SM is frequently indolent with normal life expectancy but is life threatening in patients with advanced SM. In France, midostaurin, a tyrosine kinase inhibitor, is the only approved treatment for the three advanced SM subtypes [Aggressive Systemic Mastocytosis (ASM), Systemic Mastocytosis with Associated Haemotological Neoplasms (SM-AHN) and Mast Cell Leukemia (MCL)].
Aims
This study aimed to describe in real-life the characteristics and outcomes of patients treated with midostaurin for advanced systemic mastocytosis.
Methods
A retrospective cohort study was conducted using the French National Healthcare Database (SNDS). The study population included all adults patients with advanced SM treated with midostaurin between January 1, 2012 and September 30, 2018. Kaplan Meier model was used to assess survival and competing risk survival model was used to assess treatment duration, using death as competing risk. Survival analysis was realized on a sub-group of 61 patients exclusively affiliated to the French general insurance scheme.
Results
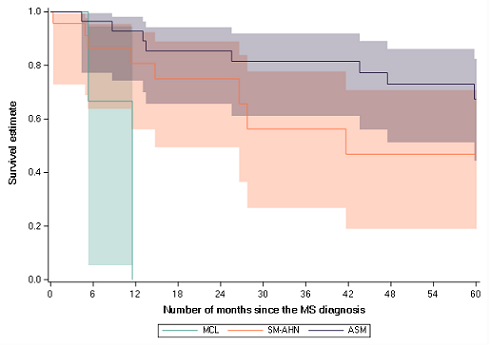
Overall, 81 patients treated with midostaurin for advanced SM were identified and their data collected, mainly from 2016 and later on (68%). The most frequent subtype was ASM (n=37), followed by SM-AHN (n=31) and MCL (n=4). The SM subtype was undetermined in 9 patients. The median age was 67 years and 64% of patients were men. Over the 11.4 months mean follow-up, median midostaurin treatment duration was 8.4 months and 28 patients were still on treatment at the end of the study. Discontinuation from the study was firstly due to a treatment discontinuation (42%) followed by the end of the study period (Dec 31, 2018, 35%), death (16%) and switch to another treatment (7%). The estimated overall survival (considering any deaths) from time since diagnosis was 83% at one year and 56% at five years. The overall survival in the different MS sub-types was statistically different (p=0.0095) with ASM>SM-AHN>MCL: 1-year overall survival was respectively 93% for ASM patients, 81% for SM-AHN patients and 0% for MCL patients. Over the follow-up period, 12 serious adverse events were identified, with the most frequent one being pneumonia (N=6 patients).
Conclusion
This study described for the first time the characteristics and outcomes of patients treated in real-life with midostaurin for advanced SM. It highlights that in routine clinical practice the characteristics of the patients, their disease management, treatment tolerance (treatment discontinuation) and survival were in line with previous published results (compassionate use and clinical trials). This study confirms the positive outcomes of midostaurin in AdvSM patients in France.
Keyword(s): Survival, Systemic mastocytosis, Treatment, Tyrosine kinase inhibitor
Abstract: EP1095
Type: E-Poster Presentation
Session title: Myeloproliferative neoplasms - Clinical
Background
Mastocytosis is a heterogeneous group of rare diseases related to the proliferation and/or abnormal accumulation of mast cells in one or more organs. The most common form, observed mostly in children, is cutaneous mastocytosis where the infiltration of mast cells is limited to the skin. When it reaches other organs, the disease is called systemic mastocytosis (SM). SM is frequently indolent with normal life expectancy but is life threatening in patients with advanced SM. In France, midostaurin, a tyrosine kinase inhibitor, is the only approved treatment for the three advanced SM subtypes [Aggressive Systemic Mastocytosis (ASM), Systemic Mastocytosis with Associated Haemotological Neoplasms (SM-AHN) and Mast Cell Leukemia (MCL)].
Aims
This study aimed to describe in real-life the characteristics and outcomes of patients treated with midostaurin for advanced systemic mastocytosis.
Methods
A retrospective cohort study was conducted using the French National Healthcare Database (SNDS). The study population included all adults patients with advanced SM treated with midostaurin between January 1, 2012 and September 30, 2018. Kaplan Meier model was used to assess survival and competing risk survival model was used to assess treatment duration, using death as competing risk. Survival analysis was realized on a sub-group of 61 patients exclusively affiliated to the French general insurance scheme.
Results
Overall, 81 patients treated with midostaurin for advanced SM were identified and their data collected, mainly from 2016 and later on (68%). The most frequent subtype was ASM (n=37), followed by SM-AHN (n=31) and MCL (n=4). The SM subtype was undetermined in 9 patients. The median age was 67 years and 64% of patients were men. Over the 11.4 months mean follow-up, median midostaurin treatment duration was 8.4 months and 28 patients were still on treatment at the end of the study. Discontinuation from the study was firstly due to a treatment discontinuation (42%) followed by the end of the study period (Dec 31, 2018, 35%), death (16%) and switch to another treatment (7%). The estimated overall survival (considering any deaths) from time since diagnosis was 83% at one year and 56% at five years. The overall survival in the different MS sub-types was statistically different (p=0.0095) with ASM>SM-AHN>MCL: 1-year overall survival was respectively 93% for ASM patients, 81% for SM-AHN patients and 0% for MCL patients. Over the follow-up period, 12 serious adverse events were identified, with the most frequent one being pneumonia (N=6 patients).

Conclusion
This study described for the first time the characteristics and outcomes of patients treated in real-life with midostaurin for advanced SM. It highlights that in routine clinical practice the characteristics of the patients, their disease management, treatment tolerance (treatment discontinuation) and survival were in line with previous published results (compassionate use and clinical trials). This study confirms the positive outcomes of midostaurin in AdvSM patients in France.
Keyword(s): Survival, Systemic mastocytosis, Treatment, Tyrosine kinase inhibitor


