
Contributions
Abstract: EP1093
Type: E-Poster Presentation
Session title: Myeloproliferative neoplasms - Clinical
Background
The clinical and molecular factors for thrombosis in myelofibrosis are not well studied.
Aims
To measure the association of molecular and clinical factors with thrombotic events in MF.
Methods
Design: Retrospective, single-centre study.
Study Population and Setting: All chronic phase MF patients from 2004 to 2018 seen at Princess Margaret Cancer Centre, Toronto, Canada.
Exposure: Our primary exposure variable was JAK2 mutation; and DNMT3A, ASXL1 and TET mutations in a subset with myeloid next generation sequencing availability. Clinical variables were age, sex, cardiovascular risk factors (smoking, hypertension, diabetes mellitus, and hypercholesterolemia), thrombosis prior to MF diagnosis, blood counts, DIPSS risk.
Outcomes and measures: The primary outcome was cumulative incidence of first thrombosis (arterial or venous) 30 days after diagnosis. The effect of covariates was measured as sub-distribution hazard ratio (SHR) using Fine-Gray method to account for competing risks from death.
Results
Total 439 MF patients were included in the study, 229 (52%) were primary, 153 (35%), secondary, and 57 (13%) pre-fibrotic. The median age was 68.6 years (SD 12.7), 59% were male. The median follow-up was 4.2 years (Range 0-18). JAK2 mutations were detected in 293 patients (66.7%), CALR in 82 (18.6%), MPL in 26 (5.9%), and 38 (8.6%) were triple negative with no canonical MPN driver mutations. 228 (52%) had next generation myeloid sequencing results: 120 (52.6%) had at least 1 Tier I/II mutation in ASXL1 (n = 83, 36.4%), EZH2 (n = 22, 9.6%), IDH1 (n = 3, 2.8%), IDH2 (n = 2, 1.8%), SRSF2 (n = 11, 10%); DNMT3A (n=18, 7.9%) or TET2 (n=68, 29.8%).
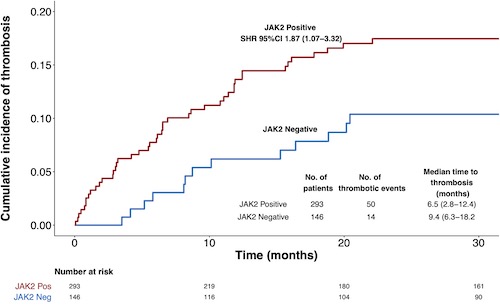
There were 64 (14.6%) thrombotic events (37 venous, 27 arterial) after diagnosis. The median time to thrombosis was 8.1 months (IQR 3.1-15.6). The 1-year cumulative incidence of thrombosis was 11.2% (95% CI 8.3-14.5) [6.6% (95% CI 4.3-8.2) arterial and 6.7% (95% CI 5.1-8.1) venous]. The incidence rate ratios for thrombosis per 100 person-years for those with JAK2 mutation compared to those without was 2.6 (1.9-4.9, p-value <0.001). The univariate Fine Gray analysis showed only JAK2 mutation and prior thrombosis were associated with increased thrombosis risk (Figure 1) and also remained significant in the multivariable model [SHR: JAK2 mutation 1.93, 95% CI 0 1.07-3.59; prior thrombosis 4.06, 95% CI 2.38-6.90]. For model validation, bootstrap cross‐validation with 1000 samples was used. The Wolber's concordance index at 1-year was 0.65, suggesting adequate discrimination. The model showed good calibration graphically.
We then studied the influence of clonal hematopoiesis associated mutations (DNMT3A, ASXL1, TET2) on thrombosis in a subset cohort of 228 patients. Patients with TET2 mutation had a significant association with thrombosis in univariate analysis; DNMT3A or ASXL1 mutations did not. In the multivariable model, TET mutations did not show an independent effect on thrombotic risk. Patients with co-occurrence of JAK2 and TET2 mutations (SHR 4.95, 95% CI 1.8-13.7) had similar thrombotic risk compared to patients with JAK2 (SHR 3.02, 95% CI 1.10-8.3) mutation alone (p-value 0.1). Prior thrombosis remained a significant variable in this model.
Conclusion
In MF, the 1-year cumulative incidence of thrombosis after diagnosis was 11.2%. JAK2 mutation and prior thrombosis independently predicted a higher risk of thrombosis. DNMT3A, TET2 or ASXL1 did not show an independent effect on risk of thrombosis. These data provide useful information to further develop thrombo-prophylaxis strategies in MF patients.
Keyword(s):
Abstract: EP1093
Type: E-Poster Presentation
Session title: Myeloproliferative neoplasms - Clinical
Background
The clinical and molecular factors for thrombosis in myelofibrosis are not well studied.
Aims
To measure the association of molecular and clinical factors with thrombotic events in MF.
Methods
Design: Retrospective, single-centre study.
Study Population and Setting: All chronic phase MF patients from 2004 to 2018 seen at Princess Margaret Cancer Centre, Toronto, Canada.
Exposure: Our primary exposure variable was JAK2 mutation; and DNMT3A, ASXL1 and TET mutations in a subset with myeloid next generation sequencing availability. Clinical variables were age, sex, cardiovascular risk factors (smoking, hypertension, diabetes mellitus, and hypercholesterolemia), thrombosis prior to MF diagnosis, blood counts, DIPSS risk.
Outcomes and measures: The primary outcome was cumulative incidence of first thrombosis (arterial or venous) 30 days after diagnosis. The effect of covariates was measured as sub-distribution hazard ratio (SHR) using Fine-Gray method to account for competing risks from death.
Results
Total 439 MF patients were included in the study, 229 (52%) were primary, 153 (35%), secondary, and 57 (13%) pre-fibrotic. The median age was 68.6 years (SD 12.7), 59% were male. The median follow-up was 4.2 years (Range 0-18). JAK2 mutations were detected in 293 patients (66.7%), CALR in 82 (18.6%), MPL in 26 (5.9%), and 38 (8.6%) were triple negative with no canonical MPN driver mutations. 228 (52%) had next generation myeloid sequencing results: 120 (52.6%) had at least 1 Tier I/II mutation in ASXL1 (n = 83, 36.4%), EZH2 (n = 22, 9.6%), IDH1 (n = 3, 2.8%), IDH2 (n = 2, 1.8%), SRSF2 (n = 11, 10%); DNMT3A (n=18, 7.9%) or TET2 (n=68, 29.8%).
There were 64 (14.6%) thrombotic events (37 venous, 27 arterial) after diagnosis. The median time to thrombosis was 8.1 months (IQR 3.1-15.6). The 1-year cumulative incidence of thrombosis was 11.2% (95% CI 8.3-14.5) [6.6% (95% CI 4.3-8.2) arterial and 6.7% (95% CI 5.1-8.1) venous]. The incidence rate ratios for thrombosis per 100 person-years for those with JAK2 mutation compared to those without was 2.6 (1.9-4.9, p-value <0.001). The univariate Fine Gray analysis showed only JAK2 mutation and prior thrombosis were associated with increased thrombosis risk (Figure 1) and also remained significant in the multivariable model [SHR: JAK2 mutation 1.93, 95% CI 0 1.07-3.59; prior thrombosis 4.06, 95% CI 2.38-6.90]. For model validation, bootstrap cross‐validation with 1000 samples was used. The Wolber's concordance index at 1-year was 0.65, suggesting adequate discrimination. The model showed good calibration graphically.
We then studied the influence of clonal hematopoiesis associated mutations (DNMT3A, ASXL1, TET2) on thrombosis in a subset cohort of 228 patients. Patients with TET2 mutation had a significant association with thrombosis in univariate analysis; DNMT3A or ASXL1 mutations did not. In the multivariable model, TET mutations did not show an independent effect on thrombotic risk. Patients with co-occurrence of JAK2 and TET2 mutations (SHR 4.95, 95% CI 1.8-13.7) had similar thrombotic risk compared to patients with JAK2 (SHR 3.02, 95% CI 1.10-8.3) mutation alone (p-value 0.1). Prior thrombosis remained a significant variable in this model.

Conclusion
In MF, the 1-year cumulative incidence of thrombosis after diagnosis was 11.2%. JAK2 mutation and prior thrombosis independently predicted a higher risk of thrombosis. DNMT3A, TET2 or ASXL1 did not show an independent effect on risk of thrombosis. These data provide useful information to further develop thrombo-prophylaxis strategies in MF patients.
Keyword(s):


