
Contributions
Abstract: EP1087
Type: E-Poster Presentation
Session title: Myeloproliferative neoplasms - Clinical
Background
Mepolizumab, an anti-interleukin-5 monoclonal antibody, has been shown to reduce disease flares and blood eosinophil counts (BEC) with a favorable safety profile in patients with uncontrolled FIP1L1-PDGFRA-negative hypereosinophilic syndrome (HES).
Aims
To further investigate the safety and efficacy of mepolizumab in patients who had previously completed the 32-week, randomized, double-blind, placebo-controlled, Phase III study, 200622 (NCT02836496).
Methods
In this open-label extension study (205203), patients who previously received mepolizumab or placebo during 200622 received 4-weekly mepolizumab 300 mg subcutaneously (SC) for 20 weeks, plus standard of care (SoC) adapted at their physicians’ discretion. All patients provided written informed consent. Investigators and patients were blinded to BEC until Week 4. Primary safety endpoints included the number of patients with adverse events (AEs), serious AEs (SAEs), AEs of special interest (AESIs), and anti-drug antibodies (ADAs). Efficacy and pharmacodynamic endpoints included the annualized rate of flares (physician-identified based on worsening symptoms/BEC), plus changes from baseline in mean daily oral corticosteroid (OCS) dose and BEC.
Results
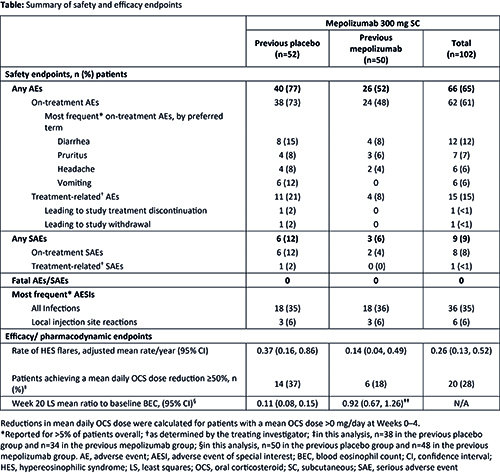
Of the 104 patients who completed 200622, 102 (98%) enrolled in 205203 (previous placebo n=52, previous mepolizumab n=50; 54% female; mean [SD] age 46.0 [15.5] years). Exposure to mepolizumab during both studies was 70.0 patient-years. Overall, 62 (61%) patients experienced on-treatment AEs (Table); the maximum severity was mild and moderate for 18% and 31% of patients, respectively. Treatment-related AEs were reported by 15 (15%) patients and one SAE was considered treatment-related; AESIs were uncommon and no events were fatal (Table). One patient was positive for ADAs at baseline (but negative thereafter) and no neutralizing antibodies were detected. The mean annualized rates of flares (95% confidence intervals) during 205203 were 0.37 (0.16, 0.86) and 0.14 (0.04, 0.49) events/year for the previous placebo and previous mepolizumab groups, respectively, versus a mean of 2.7 events for each treatment arm in the year preceding 200622. Of the 72 patients using OCS during Weeks 0–4, 20 (28%) achieved a ≥50% reduction in daily dose during Weeks 16–20 (Table). Reductions in BEC observed with mepolizumab in 200622 were maintained in 205203; for the previous placebo group, the adjusted mean reduction from baseline at Week 20 was 89%.
Conclusion
Mepolizumab 300mg SC plus SoC has a favorable safety profile without the development of neutralizing antibodies, and is associated with reductions in flares, OCS use, and BEC. These findings support previous safety and efficacy data for mepolizumab in patients with uncontrolled FIP1L1-PDGFRA-negative HES.
Funding: GSK [205203/NCT03306043]
Keyword(s): Clinical trial, Hypereosinophilic syndrome, Treatment
Abstract: EP1087
Type: E-Poster Presentation
Session title: Myeloproliferative neoplasms - Clinical
Background
Mepolizumab, an anti-interleukin-5 monoclonal antibody, has been shown to reduce disease flares and blood eosinophil counts (BEC) with a favorable safety profile in patients with uncontrolled FIP1L1-PDGFRA-negative hypereosinophilic syndrome (HES).
Aims
To further investigate the safety and efficacy of mepolizumab in patients who had previously completed the 32-week, randomized, double-blind, placebo-controlled, Phase III study, 200622 (NCT02836496).
Methods
In this open-label extension study (205203), patients who previously received mepolizumab or placebo during 200622 received 4-weekly mepolizumab 300 mg subcutaneously (SC) for 20 weeks, plus standard of care (SoC) adapted at their physicians’ discretion. All patients provided written informed consent. Investigators and patients were blinded to BEC until Week 4. Primary safety endpoints included the number of patients with adverse events (AEs), serious AEs (SAEs), AEs of special interest (AESIs), and anti-drug antibodies (ADAs). Efficacy and pharmacodynamic endpoints included the annualized rate of flares (physician-identified based on worsening symptoms/BEC), plus changes from baseline in mean daily oral corticosteroid (OCS) dose and BEC.
Results
Of the 104 patients who completed 200622, 102 (98%) enrolled in 205203 (previous placebo n=52, previous mepolizumab n=50; 54% female; mean [SD] age 46.0 [15.5] years). Exposure to mepolizumab during both studies was 70.0 patient-years. Overall, 62 (61%) patients experienced on-treatment AEs (Table); the maximum severity was mild and moderate for 18% and 31% of patients, respectively. Treatment-related AEs were reported by 15 (15%) patients and one SAE was considered treatment-related; AESIs were uncommon and no events were fatal (Table). One patient was positive for ADAs at baseline (but negative thereafter) and no neutralizing antibodies were detected. The mean annualized rates of flares (95% confidence intervals) during 205203 were 0.37 (0.16, 0.86) and 0.14 (0.04, 0.49) events/year for the previous placebo and previous mepolizumab groups, respectively, versus a mean of 2.7 events for each treatment arm in the year preceding 200622. Of the 72 patients using OCS during Weeks 0–4, 20 (28%) achieved a ≥50% reduction in daily dose during Weeks 16–20 (Table). Reductions in BEC observed with mepolizumab in 200622 were maintained in 205203; for the previous placebo group, the adjusted mean reduction from baseline at Week 20 was 89%.

Conclusion
Mepolizumab 300mg SC plus SoC has a favorable safety profile without the development of neutralizing antibodies, and is associated with reductions in flares, OCS use, and BEC. These findings support previous safety and efficacy data for mepolizumab in patients with uncontrolled FIP1L1-PDGFRA-negative HES.
Funding: GSK [205203/NCT03306043]
Keyword(s): Clinical trial, Hypereosinophilic syndrome, Treatment


