
Contributions
Abstract: EP1083
Type: E-Poster Presentation
Session title: Myeloproliferative neoplasms - Clinical
Background
JAK-inhibitor (JAKi) therapy has become standard of care for symptomatic patients with myelofibrosis. While effective in ameliorating symptoms of MF, JAKi therapy is limited by variable duration of therapeutic benefit. Survival following JAKi failure is short, and the impact of clinical and molecular factors at JAKi failure is not well studied.
Aims
To characterize the clinical and molecular factors that influence the outcomes of JAKi failure in a myelofibrosis cohort with mature follow-up.
Methods
We conducted a retrospective cohort study analyzing a well-curated dataset of 113 patients in chronic phase MF patients treated with first line ruxolitinib (n=85) or momelotinib (n=28) at the Princess Margaret Cancer Centre between 11/2009 and 02/2019. Patients were included if they had a sample available for targeted mutational profiling of clinically relevant genes in myeloid neoplasms prior to start of JAKi therapy. Paired samples were available for a subset of 55 patients at the time of JAKi failure (n=49), or after sustained clinical response >3 years (n=6). The Canadian MPN Group consensus criteria was used to define JAKi failure along with its pattern: loss or lack of spleen response, cytopenias, progression to acceleration/blast phase (AP/BP), second malignancy, or non-hematologic toxicity (Gupta et al, JCO Oncology Practice, 2020).
Results
Median follow-up in survivors was 74 (range 21-120) months. A total of 107 (95%) patients experienced JAKi failure, and cumulative incidence of JAKi failure at 1,3 and 5 years was 34%, 71%, 87%, respectively. JAKi therapy failure rates were similar for those treated with ruxolitinib or momelotinib. Median overall survival (OS) was 41.8 months after JAKi start and 13.6 months in 107 patients after first line JAKi failure. In multivariate analysis, survival after JAKi failure was associated with high DIPSS score (HR 4.06, 95%CI 1.61-10.21), ECOG ≥2 (HR 5.58, 95%CI 2.27-13.72) and transformation to AP/BP (HR 2.44 95%CI 1.04-5.7)
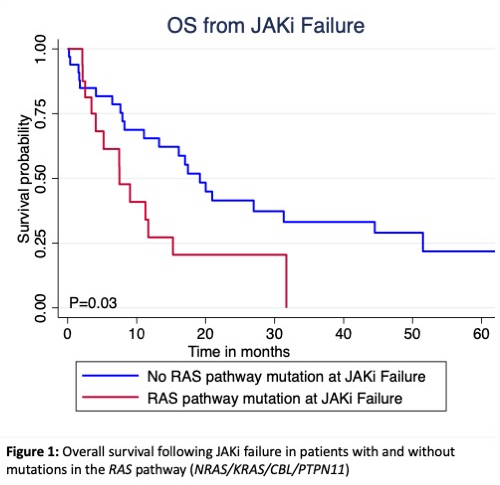
Paired samples were available for mutation analysis in 49 patients at time of failure, and 6 patients with ongoing clinical response. A total of 30 emergent mutations were observed in 20 (43%) patients with JAKi failure. The most common emergent mutations were in KRAS (n=4) and ASXL1 (n=4); with RAS pathway genes (KRAS, NRAS, CBL, and PTPN11) the most common class of emergent mutations occurring in 9 (45%) patients. RAS pathway mutations at time of JAKi failure, including persistent (n=8) and emergent (n=9) mutations, were present in: 7/23 (30%) patients with spleen progression, 5/10 (50%) with AP/BP, and 1/10 (10%) with cytopenia, 2/4 (50%) with non-heme toxicity, and 1/2 (50%) with secondary malignancy. The presence of persistent or emergent RAS pathway (P=0.03, Figure 1), TET2 (P=0.04), or high molecular risk (HMR; ASXL1, EZH2, IDH1/2, SRSF2, U2AF1 Q157)(p=0.009) mutations were associated with inferior survival.
Conclusion
Survival following first line JAKi failure is poor. Higher DIPSS/ECOG at time of failure and patients with disease transformation to AP/BP have worse survival compared to other types of JAKi therapy failure. RAS pathway gene mutations were the most common emergent mutations at JAKi therapy failure. The presence of persistent or emergent RAS pathway (Figure 1), TET2, or HMR mutations appear to predict inferior survival following JAKi failure.
Keyword(s): Clinical outcome, Janus Kinase inhibitor, Mutation status, Myeloproliferative disorder
Abstract: EP1083
Type: E-Poster Presentation
Session title: Myeloproliferative neoplasms - Clinical
Background
JAK-inhibitor (JAKi) therapy has become standard of care for symptomatic patients with myelofibrosis. While effective in ameliorating symptoms of MF, JAKi therapy is limited by variable duration of therapeutic benefit. Survival following JAKi failure is short, and the impact of clinical and molecular factors at JAKi failure is not well studied.
Aims
To characterize the clinical and molecular factors that influence the outcomes of JAKi failure in a myelofibrosis cohort with mature follow-up.
Methods
We conducted a retrospective cohort study analyzing a well-curated dataset of 113 patients in chronic phase MF patients treated with first line ruxolitinib (n=85) or momelotinib (n=28) at the Princess Margaret Cancer Centre between 11/2009 and 02/2019. Patients were included if they had a sample available for targeted mutational profiling of clinically relevant genes in myeloid neoplasms prior to start of JAKi therapy. Paired samples were available for a subset of 55 patients at the time of JAKi failure (n=49), or after sustained clinical response >3 years (n=6). The Canadian MPN Group consensus criteria was used to define JAKi failure along with its pattern: loss or lack of spleen response, cytopenias, progression to acceleration/blast phase (AP/BP), second malignancy, or non-hematologic toxicity (Gupta et al, JCO Oncology Practice, 2020).
Results
Median follow-up in survivors was 74 (range 21-120) months. A total of 107 (95%) patients experienced JAKi failure, and cumulative incidence of JAKi failure at 1,3 and 5 years was 34%, 71%, 87%, respectively. JAKi therapy failure rates were similar for those treated with ruxolitinib or momelotinib. Median overall survival (OS) was 41.8 months after JAKi start and 13.6 months in 107 patients after first line JAKi failure. In multivariate analysis, survival after JAKi failure was associated with high DIPSS score (HR 4.06, 95%CI 1.61-10.21), ECOG ≥2 (HR 5.58, 95%CI 2.27-13.72) and transformation to AP/BP (HR 2.44 95%CI 1.04-5.7)
Paired samples were available for mutation analysis in 49 patients at time of failure, and 6 patients with ongoing clinical response. A total of 30 emergent mutations were observed in 20 (43%) patients with JAKi failure. The most common emergent mutations were in KRAS (n=4) and ASXL1 (n=4); with RAS pathway genes (KRAS, NRAS, CBL, and PTPN11) the most common class of emergent mutations occurring in 9 (45%) patients. RAS pathway mutations at time of JAKi failure, including persistent (n=8) and emergent (n=9) mutations, were present in: 7/23 (30%) patients with spleen progression, 5/10 (50%) with AP/BP, and 1/10 (10%) with cytopenia, 2/4 (50%) with non-heme toxicity, and 1/2 (50%) with secondary malignancy. The presence of persistent or emergent RAS pathway (P=0.03, Figure 1), TET2 (P=0.04), or high molecular risk (HMR; ASXL1, EZH2, IDH1/2, SRSF2, U2AF1 Q157)(p=0.009) mutations were associated with inferior survival.

Conclusion
Survival following first line JAKi failure is poor. Higher DIPSS/ECOG at time of failure and patients with disease transformation to AP/BP have worse survival compared to other types of JAKi therapy failure. RAS pathway gene mutations were the most common emergent mutations at JAKi therapy failure. The presence of persistent or emergent RAS pathway (Figure 1), TET2, or HMR mutations appear to predict inferior survival following JAKi failure.
Keyword(s): Clinical outcome, Janus Kinase inhibitor, Mutation status, Myeloproliferative disorder


