
Contributions
Abstract: EP1079
Type: E-Poster Presentation
Session title: Myeloproliferative neoplasms - Clinical
Background
In intermediate (int)-2/high-risk patients (pts) with myelofibrosis (MF), presence of ≥3 high molecular risk (HMR) mutations (IDH1/2, ASXL-1, SRSF2, EZH2) was associated with lower response rates to ruxolitinib (RUX) (Newberry, Blood 2016). Although RUX is widely used in int-1 risk pts, the impact of HMR on response and outcome is unknown in this setting.
Aims
To investigate the impact of HMR mutations on treatment success and outcome in int-1 risk RUX-treated MF pts.
Methods
After IRB approval, the “RUX-MF” retrospective study collected 739 RUX-treated MF pts in 25 Hematology Centers. Overall, 361 (48.9%) pts received RUX while at int-1 risk according to DIPSS (primary MF, PMF) or to MYSEC-PM (secondary MF, SMF). In 79 int-1 pts, HMR status was evaluated by next generation sequencing (NGS) before or soon after RUX start. Clinical/laboratory characteristics and RUX starting dose were comparable in these 79 pts and in the 282 with no HMR data available. Spleen (SR) and symptoms (SyR) response were evaluated according to IWG-MRT criteria. Overall survival/RUX stop/blast phase (BP)/infection-free survival were estimated from RUX start to death/RUX stop/BP/infection or last contact and compared with the log-rank test.
Results
The characteristics of the 79 int-1 MF pts at RUX start were: median age 65.7y (24-83); males 55.7%; PMF 44.3%; JAK2, CALR and MPL mutated: 73.4%, 25.3% and 0 (1.3% triple negative), Hb<10 g/dl: 12.7%, PLT>200 x109/l: 76%; blast cells≥1%: 33%; spleen length >10 cm: 44.3%, TSS >20: 56.5%, time from MF diagnosis to RUX start >2y in 46.8%. Starting and cumulative RUX dose >10 mg BID: 74.4% and 57.9%, respectively. Overall, ≥1 HMR was detected in 39 pts (49.4%) (≥2HMR in 12 pts). Specifically, ASXL-1 was found in 33 pts, IDH1 in 5, IDH2 in 3, SRSF2 in 6 and EZH2 in 7. While SRSF2 mutations were detected only in PMF (p=0.005), distributions of the other HMR mutations were comparable in PMF and SMF. HMR pts started RUX more frequently with large spleen (p=0.03) and lower PLT count (p=0.04) compared to no-HMR pts.
At 3 and 6 mos, 26.9% and 31.3% of pts achieved a SR, while 65.6% and 75% were in SyR, respectively. SR was less frequently achieved by HMR pts at both 3 (11.4% vs 43.8%, p=0.003) and 6 mos (18.8% vs 43.8%, p=0.03). SyR was not influenced by HMR status.
PLT count at 3 and 6 mos was always >50 x109/l in all cases but two. At 3 and 6 mos, in 33.3% and 29% of transfusion independent pts, Hb decreased <10 g/dl, while 18.7% and 19.7% became transfusion-dependent, respectively, regardless of HMR. At least one infection, mainly including lung (32.8%), urinary tract (11.7%) and gastrointestinal tract (7.6%) occurred in 28 pts, regardless of HMR (log-rank p=0.13).
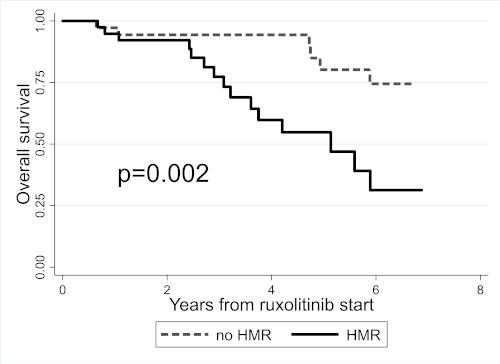
After a median RUX exposure of 2.7y (0.1-7.7), 36 (45.6%) pts discontinued RUX, 4 (5.2%) progressed to BP and 22 (27.9%) died. In HMR pts, RUX discontinuation (38.9% vs 20.8% at 3y, log-rank p=0.01) and BP progression (10% vs 0 at 3y, log-rank p=0.04) were significantly higher. Overall survival was also significantly shorter for HMR pts (log-rank p=0.002) (Fig.1).
Conclusion
In int-1 pts treated with RUX, HMR mutations are associated with lower responses, increased BP progression and worse survival. HMR evaluation is crucial for personalized management of these pts.
Keyword(s): Mutation, Myelofibrosis, Prognosis, Ruxolitinib
Abstract: EP1079
Type: E-Poster Presentation
Session title: Myeloproliferative neoplasms - Clinical
Background
In intermediate (int)-2/high-risk patients (pts) with myelofibrosis (MF), presence of ≥3 high molecular risk (HMR) mutations (IDH1/2, ASXL-1, SRSF2, EZH2) was associated with lower response rates to ruxolitinib (RUX) (Newberry, Blood 2016). Although RUX is widely used in int-1 risk pts, the impact of HMR on response and outcome is unknown in this setting.
Aims
To investigate the impact of HMR mutations on treatment success and outcome in int-1 risk RUX-treated MF pts.
Methods
After IRB approval, the “RUX-MF” retrospective study collected 739 RUX-treated MF pts in 25 Hematology Centers. Overall, 361 (48.9%) pts received RUX while at int-1 risk according to DIPSS (primary MF, PMF) or to MYSEC-PM (secondary MF, SMF). In 79 int-1 pts, HMR status was evaluated by next generation sequencing (NGS) before or soon after RUX start. Clinical/laboratory characteristics and RUX starting dose were comparable in these 79 pts and in the 282 with no HMR data available. Spleen (SR) and symptoms (SyR) response were evaluated according to IWG-MRT criteria. Overall survival/RUX stop/blast phase (BP)/infection-free survival were estimated from RUX start to death/RUX stop/BP/infection or last contact and compared with the log-rank test.
Results
The characteristics of the 79 int-1 MF pts at RUX start were: median age 65.7y (24-83); males 55.7%; PMF 44.3%; JAK2, CALR and MPL mutated: 73.4%, 25.3% and 0 (1.3% triple negative), Hb<10 g/dl: 12.7%, PLT>200 x109/l: 76%; blast cells≥1%: 33%; spleen length >10 cm: 44.3%, TSS >20: 56.5%, time from MF diagnosis to RUX start >2y in 46.8%. Starting and cumulative RUX dose >10 mg BID: 74.4% and 57.9%, respectively. Overall, ≥1 HMR was detected in 39 pts (49.4%) (≥2HMR in 12 pts). Specifically, ASXL-1 was found in 33 pts, IDH1 in 5, IDH2 in 3, SRSF2 in 6 and EZH2 in 7. While SRSF2 mutations were detected only in PMF (p=0.005), distributions of the other HMR mutations were comparable in PMF and SMF. HMR pts started RUX more frequently with large spleen (p=0.03) and lower PLT count (p=0.04) compared to no-HMR pts.
At 3 and 6 mos, 26.9% and 31.3% of pts achieved a SR, while 65.6% and 75% were in SyR, respectively. SR was less frequently achieved by HMR pts at both 3 (11.4% vs 43.8%, p=0.003) and 6 mos (18.8% vs 43.8%, p=0.03). SyR was not influenced by HMR status.
PLT count at 3 and 6 mos was always >50 x109/l in all cases but two. At 3 and 6 mos, in 33.3% and 29% of transfusion independent pts, Hb decreased <10 g/dl, while 18.7% and 19.7% became transfusion-dependent, respectively, regardless of HMR. At least one infection, mainly including lung (32.8%), urinary tract (11.7%) and gastrointestinal tract (7.6%) occurred in 28 pts, regardless of HMR (log-rank p=0.13).
After a median RUX exposure of 2.7y (0.1-7.7), 36 (45.6%) pts discontinued RUX, 4 (5.2%) progressed to BP and 22 (27.9%) died. In HMR pts, RUX discontinuation (38.9% vs 20.8% at 3y, log-rank p=0.01) and BP progression (10% vs 0 at 3y, log-rank p=0.04) were significantly higher. Overall survival was also significantly shorter for HMR pts (log-rank p=0.002) (Fig.1).

Conclusion
In int-1 pts treated with RUX, HMR mutations are associated with lower responses, increased BP progression and worse survival. HMR evaluation is crucial for personalized management of these pts.
Keyword(s): Mutation, Myelofibrosis, Prognosis, Ruxolitinib


