
Contributions
Abstract: EP1078
Type: E-Poster Presentation
Session title: Myeloproliferative neoplasms - Clinical
Background
Navitoclax (Nav) is an oral small-molecule inhibitor of antiapoptotic B-cell lymphoma 2 proteins (BCL-XL, BCL-2, BCL-W) that has shown pronounced antitumor activity in xenograft models. Interim analyses of an ongoing, multicenter, Phase 2 trial (NCT03222609) of Nav plus the Janus kinase 1/2 (JAK1/2) inhibitor ruxolitinib (Rux) in 34 patients (pts) with myelofibrosis (MF) were previously reported (Pemmaraju et al. ASH 2020, #52).
Aims
To provide an update on the findings from this Phase 2 trial of Nav plus Rux in pts with MF.
Methods
Pts with primary or secondary MF who had previously received Rux for ≥12 weeks continued their current stable dose of Rux, and Nav was initiated at 50 mg/day and escalated to a maximum of 300 mg/day, as tolerated based on platelet count. The primary endpoint was spleen volume reduction of ≥35% (SVR35) from baseline (BL) at Week (Wk) 24. Secondary endpoints included improvement in total symptom score (TSS, as measured by the Myelofibrosis Symptom Assessment Form v4.0), bone marrow fibrosis (BMF), anemia response (per International Working Group [IWG] criteria), and safety. Exploratory endpoints were duration of response (DOR) of SVR35, and overall survival (OS).
Results
At the data cutoff, August 30, 2020, 34 pts with MF received ≥1 dose of Nav plus Rux. Median age was 68 years (range 42–86). At BL, the median spleen volume was 1,695 cm3 (range 465–5,047). At BL, 9 pts were transfusion independent (TI) with hemoglobin (Hbg) <10g/dL and 2 pts were transfusion dependent per IWG criteria. Of 33 pts with BL testing, 79% and 21% had JAK2 and CALR mutations (4/7 CALR type 1, 3/7 CALR type 2), respectively, and 19 pts had high molecular risk mutations (HMR); 17 pts had ≥3 genes mutated at enrollment. Median duration of Rux exposure prior to study was 82 wks (range 19–308).
Twenty-four (71%) pts received the maximum Nav dose of 300 mg/day. Median Nav exposure since study onset was 81 wks (range 4–126); 24 pts remain on-study and 17 pts remain on-treatment. Of 17 pts who discontinued Nav, the most common reasons were progressive disease (29%) and adverse events (AEs, 18%).
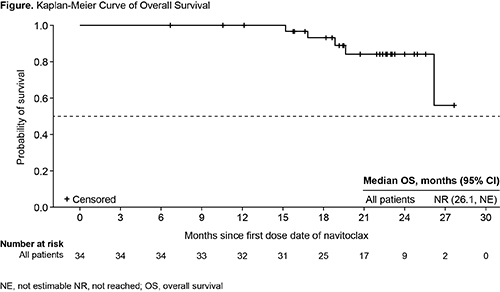
SVR35 from BL to Wk 24 was reported for 9 (27%) pts, independent of HMR, and SVR35 at any time was achieved in 15 (44%) pts; responses were seen at wk 12 in 6 (18%) pts. Median DOR of SVR35 was 13.8 months (95% confidence interval [CI]: 8.2–not reached), which was similar in pts with and without HMR. TSS was reduced by ≥50% in 6/20 (30%) pts who were evaluable at Wk 24. BMF improved by ≥1 grade at any time on study in 11/33 (33%) pts. Hgb levels improved on-study; 7/11 (64%) pts with Hgb <10 g/dL or transfusion dependency at BL had improvement in Hgb of ≥2 g/dL (n=6) or became TI (n=1). At a median follow-up of 105 wks, median OS was not reached (Figure); OS estimate at 24 months was 84% (95% CI: 63.0%–93.9%).
All pts experienced an AE, most common AEs were thrombocytopenia (88%), diarrhea (71%), and fatigue (62%), with most gastrointestinal AEs of Grade 1/2. Thirty (88%) pts experienced a Grade 3/4 AE, and 15 (44%) had a serious AE (SAE). The most common Grade ≥3 AEs were thrombocytopenia (without clinically significant bleeding; 56%) and anemia (32%), and the most common SAE was pneumonia (12%). Thrombocytopenia led to Nav dose reduction in 19 (56%) pts.
Conclusion
SVR was durable and encouraging, and improvement in TSS, anemia response, BMF, and OS were clinically meaningful. These data suggest that Nav plus Rux may exert disease-modifying activity in MF and warrant further testing in Phase 3 clinical studies.
Keyword(s): BCL2, Janus Kinase inhibitor, Myelofibrosis, Outcome
Abstract: EP1078
Type: E-Poster Presentation
Session title: Myeloproliferative neoplasms - Clinical
Background
Navitoclax (Nav) is an oral small-molecule inhibitor of antiapoptotic B-cell lymphoma 2 proteins (BCL-XL, BCL-2, BCL-W) that has shown pronounced antitumor activity in xenograft models. Interim analyses of an ongoing, multicenter, Phase 2 trial (NCT03222609) of Nav plus the Janus kinase 1/2 (JAK1/2) inhibitor ruxolitinib (Rux) in 34 patients (pts) with myelofibrosis (MF) were previously reported (Pemmaraju et al. ASH 2020, #52).
Aims
To provide an update on the findings from this Phase 2 trial of Nav plus Rux in pts with MF.
Methods
Pts with primary or secondary MF who had previously received Rux for ≥12 weeks continued their current stable dose of Rux, and Nav was initiated at 50 mg/day and escalated to a maximum of 300 mg/day, as tolerated based on platelet count. The primary endpoint was spleen volume reduction of ≥35% (SVR35) from baseline (BL) at Week (Wk) 24. Secondary endpoints included improvement in total symptom score (TSS, as measured by the Myelofibrosis Symptom Assessment Form v4.0), bone marrow fibrosis (BMF), anemia response (per International Working Group [IWG] criteria), and safety. Exploratory endpoints were duration of response (DOR) of SVR35, and overall survival (OS).
Results
At the data cutoff, August 30, 2020, 34 pts with MF received ≥1 dose of Nav plus Rux. Median age was 68 years (range 42–86). At BL, the median spleen volume was 1,695 cm3 (range 465–5,047). At BL, 9 pts were transfusion independent (TI) with hemoglobin (Hbg) <10g/dL and 2 pts were transfusion dependent per IWG criteria. Of 33 pts with BL testing, 79% and 21% had JAK2 and CALR mutations (4/7 CALR type 1, 3/7 CALR type 2), respectively, and 19 pts had high molecular risk mutations (HMR); 17 pts had ≥3 genes mutated at enrollment. Median duration of Rux exposure prior to study was 82 wks (range 19–308).
Twenty-four (71%) pts received the maximum Nav dose of 300 mg/day. Median Nav exposure since study onset was 81 wks (range 4–126); 24 pts remain on-study and 17 pts remain on-treatment. Of 17 pts who discontinued Nav, the most common reasons were progressive disease (29%) and adverse events (AEs, 18%).
SVR35 from BL to Wk 24 was reported for 9 (27%) pts, independent of HMR, and SVR35 at any time was achieved in 15 (44%) pts; responses were seen at wk 12 in 6 (18%) pts. Median DOR of SVR35 was 13.8 months (95% confidence interval [CI]: 8.2–not reached), which was similar in pts with and without HMR. TSS was reduced by ≥50% in 6/20 (30%) pts who were evaluable at Wk 24. BMF improved by ≥1 grade at any time on study in 11/33 (33%) pts. Hgb levels improved on-study; 7/11 (64%) pts with Hgb <10 g/dL or transfusion dependency at BL had improvement in Hgb of ≥2 g/dL (n=6) or became TI (n=1). At a median follow-up of 105 wks, median OS was not reached (Figure); OS estimate at 24 months was 84% (95% CI: 63.0%–93.9%).
All pts experienced an AE, most common AEs were thrombocytopenia (88%), diarrhea (71%), and fatigue (62%), with most gastrointestinal AEs of Grade 1/2. Thirty (88%) pts experienced a Grade 3/4 AE, and 15 (44%) had a serious AE (SAE). The most common Grade ≥3 AEs were thrombocytopenia (without clinically significant bleeding; 56%) and anemia (32%), and the most common SAE was pneumonia (12%). Thrombocytopenia led to Nav dose reduction in 19 (56%) pts.

Conclusion
SVR was durable and encouraging, and improvement in TSS, anemia response, BMF, and OS were clinically meaningful. These data suggest that Nav plus Rux may exert disease-modifying activity in MF and warrant further testing in Phase 3 clinical studies.
Keyword(s): BCL2, Janus Kinase inhibitor, Myelofibrosis, Outcome


