
Contributions
Abstract: EP1077
Type: E-Poster Presentation
Session title: Myeloproliferative neoplasms - Clinical
Background
Pelabresib (CPI-0610), a first-in-class, oral, small-molecule inhibitor of bromodomain and extraterminal domain (BET) proteins, has the potential to promote disease-modifying activity through altered gene regulation of key oncogenic, fibrotic, and inflammatory factors in the clonal disease cells of origin in myelofibrosis (MF). Furthermore, pelabresib promotes maturation and ex vivo differentiation of erythroid progenitors (Mertz, ASH 2020). Many MF patients (pts) treated with ruxolitinib (rux) develop worsening anemia and may become red blood cell (RBC) transfusion-dependent (TD) which represents a significant unmet medical need. Here we report improvement of anemia in advanced MF pts as evidenced by achievement of RBC-transfusion independence (TI) in TD pts or sustained mean hemoglobin (Hgb) increase of ≥1.5g/dL for 12 wks in non-transfusion dependent (non-TD) pts.
Aims
Evaluation of pelabresib monotherapy or as add-on to rux in advanced MF pts.
Methods
MANIFEST is an ongoing, open-label Phase 2 study. In Arm 1, pts that were refractory, intolerant or ineligible for JAKi were treated with pelabresib monotherapy. A washout of ≥2 wks since last systemic MF therapy was required. In Arm 2, pts receiving rux but not deriving adequate benefit were treated with pelabresib add-on to rux. Pts are stratified to cohorts 1A and 2A if they were TD (receiving ≥6U RBCs/12 wks) and into cohorts 1B and 2B as non-TD if they did not meet TD criteria. Primary endpoint was achievement of TI ≥12 wks in TD cohorts; ≥35% spleen volume reduction at wk 24 in non-TD cohorts.
Results
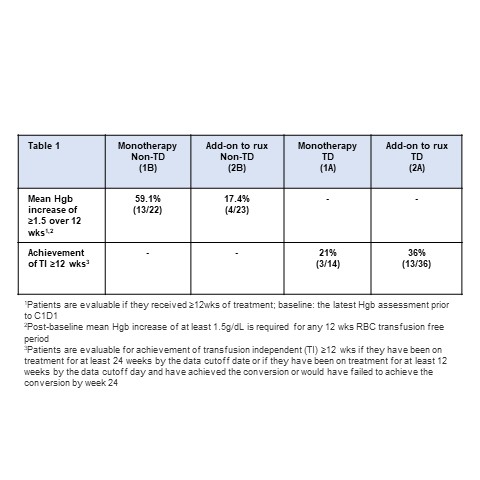
As of 29 Sep 2020, 19 TD pts and 27 non-TD pts were treated in Arm 1 (monotherapy). 76% of pts had baseline Hgb <10g/dL (Median: 9, range: 6-15). Median number of prior therapies was 2 (1-6). Median time since last rux dose in non-TD cohort was 9.2 mo (range 0.5-46 mo). In TD cohort, 21% (3/14) of pts achieved TI (median duration of transfusion-free period: 44 wk, range 32-50). In non-TD cohort, mean Hgb increase ≥ 1.5 g/dL sustained over a 12-wk transfusion-free period was achieved by 59.1% (13/22) of pts among which only 1 pt received rux within 4 months of study entry, suggesting the increase in Hgb was not a consequence of rebound due to rux discontinuation.
In Arm 2, 52 TD pts and 26 non-TD pts were treated with pelabresib as add-on to rux. 76% of pts had baseline Hgb <10g/dL (median: 9, range: 6-13). In TD cohort, 36% (13/36) of pts achieved TI (median duration of transfusion-free period: 39 wk, range 18-148). 17.4 % (4/23) of non-TD pts had mean Hgb increase ≥ 1.5 g/dL sustained over a 12-wks transfusion-free period.
In pts with an anemia response, the observed Hgb improvement or achievement of TI has been generally associated with an increase in reticulocyte count and/or increased CD71+ progenitor cells in the bone marrow, suggesting a positive pelabresib effect on erythroid differentiation.
Pelabresib was generally well tolerated. The most common treatment emergent adverse events include diarrhea (46%, ≥Gr3: 4%), thrombocytopenia (40%, ≥Gr3: 22%), nausea (36%, ≥Gr3: 2%), asthenic conditions (32%, ≥Gr3: 2%), respiratory tract infections (32%, ≥Gr3: 4% ), dysgeusia (26%, ≥Gr3: 1%) and cough (25%, no ≥Gr3).
Conclusion
Pelabresib monotherapy was associated with a mean increase in Hgb ≥ 1.5 g/dL in majority of non-TD pts and conversion of one-fifth of TD pts to TI in heavily pretreated MF pts in Arm 1. Addition of pelabresib to rux in Arm 2 pts with suboptimal response on stable doses of rux resulted in mean increase in Hgb ≥ 1.5 g/dL in 17% of non-TD pts and conversion to TI in more than one-third of TD pts.
Keyword(s): Anemia, Hemoglobin, Myelofibrosis, Ruxolitinib
Abstract: EP1077
Type: E-Poster Presentation
Session title: Myeloproliferative neoplasms - Clinical
Background
Pelabresib (CPI-0610), a first-in-class, oral, small-molecule inhibitor of bromodomain and extraterminal domain (BET) proteins, has the potential to promote disease-modifying activity through altered gene regulation of key oncogenic, fibrotic, and inflammatory factors in the clonal disease cells of origin in myelofibrosis (MF). Furthermore, pelabresib promotes maturation and ex vivo differentiation of erythroid progenitors (Mertz, ASH 2020). Many MF patients (pts) treated with ruxolitinib (rux) develop worsening anemia and may become red blood cell (RBC) transfusion-dependent (TD) which represents a significant unmet medical need. Here we report improvement of anemia in advanced MF pts as evidenced by achievement of RBC-transfusion independence (TI) in TD pts or sustained mean hemoglobin (Hgb) increase of ≥1.5g/dL for 12 wks in non-transfusion dependent (non-TD) pts.
Aims
Evaluation of pelabresib monotherapy or as add-on to rux in advanced MF pts.
Methods
MANIFEST is an ongoing, open-label Phase 2 study. In Arm 1, pts that were refractory, intolerant or ineligible for JAKi were treated with pelabresib monotherapy. A washout of ≥2 wks since last systemic MF therapy was required. In Arm 2, pts receiving rux but not deriving adequate benefit were treated with pelabresib add-on to rux. Pts are stratified to cohorts 1A and 2A if they were TD (receiving ≥6U RBCs/12 wks) and into cohorts 1B and 2B as non-TD if they did not meet TD criteria. Primary endpoint was achievement of TI ≥12 wks in TD cohorts; ≥35% spleen volume reduction at wk 24 in non-TD cohorts.
Results
As of 29 Sep 2020, 19 TD pts and 27 non-TD pts were treated in Arm 1 (monotherapy). 76% of pts had baseline Hgb <10g/dL (Median: 9, range: 6-15). Median number of prior therapies was 2 (1-6). Median time since last rux dose in non-TD cohort was 9.2 mo (range 0.5-46 mo). In TD cohort, 21% (3/14) of pts achieved TI (median duration of transfusion-free period: 44 wk, range 32-50). In non-TD cohort, mean Hgb increase ≥ 1.5 g/dL sustained over a 12-wk transfusion-free period was achieved by 59.1% (13/22) of pts among which only 1 pt received rux within 4 months of study entry, suggesting the increase in Hgb was not a consequence of rebound due to rux discontinuation.
In Arm 2, 52 TD pts and 26 non-TD pts were treated with pelabresib as add-on to rux. 76% of pts had baseline Hgb <10g/dL (median: 9, range: 6-13). In TD cohort, 36% (13/36) of pts achieved TI (median duration of transfusion-free period: 39 wk, range 18-148). 17.4 % (4/23) of non-TD pts had mean Hgb increase ≥ 1.5 g/dL sustained over a 12-wks transfusion-free period.
In pts with an anemia response, the observed Hgb improvement or achievement of TI has been generally associated with an increase in reticulocyte count and/or increased CD71+ progenitor cells in the bone marrow, suggesting a positive pelabresib effect on erythroid differentiation.
Pelabresib was generally well tolerated. The most common treatment emergent adverse events include diarrhea (46%, ≥Gr3: 4%), thrombocytopenia (40%, ≥Gr3: 22%), nausea (36%, ≥Gr3: 2%), asthenic conditions (32%, ≥Gr3: 2%), respiratory tract infections (32%, ≥Gr3: 4% ), dysgeusia (26%, ≥Gr3: 1%) and cough (25%, no ≥Gr3).

Conclusion
Pelabresib monotherapy was associated with a mean increase in Hgb ≥ 1.5 g/dL in majority of non-TD pts and conversion of one-fifth of TD pts to TI in heavily pretreated MF pts in Arm 1. Addition of pelabresib to rux in Arm 2 pts with suboptimal response on stable doses of rux resulted in mean increase in Hgb ≥ 1.5 g/dL in 17% of non-TD pts and conversion to TI in more than one-third of TD pts.
Keyword(s): Anemia, Hemoglobin, Myelofibrosis, Ruxolitinib


