
Contributions
Abstract: EP1075
Type: E-Poster Presentation
Session title: Myeloproliferative neoplasms - Clinical
Background
Ruxolitinib (rux) is highly effective in myelofibrosis (MF). Rux improves symptoms, reduces spleen size, and prolongs survival; however, suboptimal response may occur in a subset of patients (pts), potentially due to persistent PI3K/AKT pathway activation despite continued JAK inhibition. Parsaclisib (INCB050465) is a potent and highly selective next-generation PI3Kδ inhibitor. We hypothesized that adding parsaclisib would improve outcomes in pts with suboptimal response to stable rux treatment.
Aims
This phase 2 study (NCT02718300) evaluated optimal dosing, efficacy, and safety of add-on parsaclisib in pts with MF with a suboptimal response to rux.
Methods
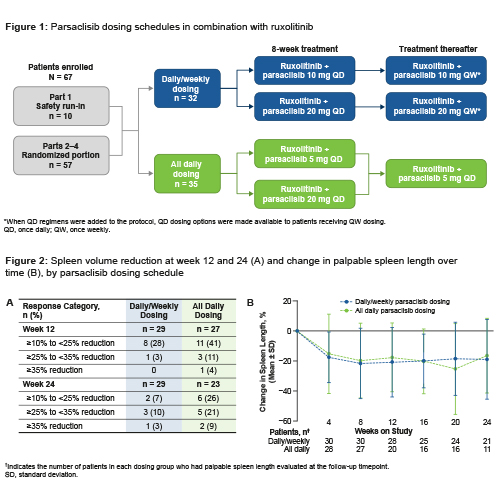
Pts had primary, post–polycythemia vera, or post–essential thrombocythemia MF, ECOG performance status ≤2, and suboptimal response (palpable spleen >10 cm below left subcostal margin [LSM]; or palpable splenomegaly 5–10 cm below LSM and presence of 1 symptom score ≥5 or 2 symptom scores ≥3 each using the Screening Symptom Form) after ≥6 months of rux (5–25 mg twice daily; rux stable dose, ≥8 weeks [wks]). Pts remained on their stable rux dose and were randomized to receive add-on parsaclisib 10 mg or 20 mg once-daily (QD) for 8 wks and the same dose once-weekly (QW) thereafter (QD/QW group), or parsaclisib 5 mg or 20 mg QD for 8 wks and 5 mg QD thereafter (all QD group) (Figure 1). The primary endpoint was change in spleen volume from baseline to wk 12 by MRI/CT scan; other endpoints included change in spleen length and symptoms (Myelofibrosis-Symptoms Assessment Form Total Symptom Score [MFSAF-TSS]).
Results
At data cutoff (Aug 27, 2020) for this interim analysis, 32 pts received parsaclisib QD/QW and 35 received parsaclisib all QD. Median treatment duration was 213 days; median average daily dose was 5.0 mg/day for parsaclisib and 30.0 mg/day for rux. Baseline median spleen volume (cm3) was 2414 in QD/QW (n=29) and 1885 in QD (n=29); median MFSAF-TSS was 10.8 (n=28) and 16.3 (n=26), respectively. Median percentage change in spleen volume at wk 12 was −1.9 (n=29) in QD/QW and −13.0 (n=27) in QD, and at wk 24 was −3.5 (n=23) and −21.8 (n=17), respectively (Figure 2: [A] spleen volume reduction; [B] palpable spleen length change). Median percentage change in MFSAF-TSS at wk 12 was −14.0 (n=21) in QD/QW and −37.4 (n=17) in QD.
Nonhematologic treatment-emergent adverse events (TEAEs) were primarily grade 1/2; grade 3/4 nonhematologic TEAEs occurring in >1 pt overall were urinary tract infection (n=3; all QD/QW), fall, increased alanine aminotransferase, increased aspartate aminotransferase, hypocalcemia, and hypertension (each n=2; all QD/QW), dyspnea (n=2; both QD), and fatigue and pneumonia (each n=2; each 1 QD/QW, 1 QD). New-onset grade 3 thrombocytopenia was observed in 6/32 (19%) pts in QD/QW and 9/35 (26%) pts in QD; grade 4 thrombocytopenia was observed in 6/32 (19%) pts in QD/QW and 1/35 (3%) pts in QD. TEAEs of special interest included grade ≥2 diarrhea (n=4) and grade ≥2 rash (n=1) (all QD/QW). No colitis was reported. TEAEs led to parsaclisib interruption in 18/32 pts in QD/QW and 18/35 pts in QD, and rux interruption in 6/32 and 8/35 pts, respectively; and to parsaclisib discontinuation in 7/32 pts in QD/QW and 3/35 pts in QD, and rux discontinuation in 2/32 and 1/35 pts, respectively.
Conclusion
Add-on parsaclisib showed preliminary efficacy in pts with MF experiencing suboptimal response to rux; QD dosing appeared more efficacious than QD/QW dosing. Combination therapy was generally well tolerated with limited grade 3/4 AEs and TEAE-related discontinuations.
Keyword(s): Myelofibrosis, Phase II, PI3K, Ruxolitinib
Abstract: EP1075
Type: E-Poster Presentation
Session title: Myeloproliferative neoplasms - Clinical
Background
Ruxolitinib (rux) is highly effective in myelofibrosis (MF). Rux improves symptoms, reduces spleen size, and prolongs survival; however, suboptimal response may occur in a subset of patients (pts), potentially due to persistent PI3K/AKT pathway activation despite continued JAK inhibition. Parsaclisib (INCB050465) is a potent and highly selective next-generation PI3Kδ inhibitor. We hypothesized that adding parsaclisib would improve outcomes in pts with suboptimal response to stable rux treatment.
Aims
This phase 2 study (NCT02718300) evaluated optimal dosing, efficacy, and safety of add-on parsaclisib in pts with MF with a suboptimal response to rux.
Methods
Pts had primary, post–polycythemia vera, or post–essential thrombocythemia MF, ECOG performance status ≤2, and suboptimal response (palpable spleen >10 cm below left subcostal margin [LSM]; or palpable splenomegaly 5–10 cm below LSM and presence of 1 symptom score ≥5 or 2 symptom scores ≥3 each using the Screening Symptom Form) after ≥6 months of rux (5–25 mg twice daily; rux stable dose, ≥8 weeks [wks]). Pts remained on their stable rux dose and were randomized to receive add-on parsaclisib 10 mg or 20 mg once-daily (QD) for 8 wks and the same dose once-weekly (QW) thereafter (QD/QW group), or parsaclisib 5 mg or 20 mg QD for 8 wks and 5 mg QD thereafter (all QD group) (Figure 1). The primary endpoint was change in spleen volume from baseline to wk 12 by MRI/CT scan; other endpoints included change in spleen length and symptoms (Myelofibrosis-Symptoms Assessment Form Total Symptom Score [MFSAF-TSS]).
Results
At data cutoff (Aug 27, 2020) for this interim analysis, 32 pts received parsaclisib QD/QW and 35 received parsaclisib all QD. Median treatment duration was 213 days; median average daily dose was 5.0 mg/day for parsaclisib and 30.0 mg/day for rux. Baseline median spleen volume (cm3) was 2414 in QD/QW (n=29) and 1885 in QD (n=29); median MFSAF-TSS was 10.8 (n=28) and 16.3 (n=26), respectively. Median percentage change in spleen volume at wk 12 was −1.9 (n=29) in QD/QW and −13.0 (n=27) in QD, and at wk 24 was −3.5 (n=23) and −21.8 (n=17), respectively (Figure 2: [A] spleen volume reduction; [B] palpable spleen length change). Median percentage change in MFSAF-TSS at wk 12 was −14.0 (n=21) in QD/QW and −37.4 (n=17) in QD.
Nonhematologic treatment-emergent adverse events (TEAEs) were primarily grade 1/2; grade 3/4 nonhematologic TEAEs occurring in >1 pt overall were urinary tract infection (n=3; all QD/QW), fall, increased alanine aminotransferase, increased aspartate aminotransferase, hypocalcemia, and hypertension (each n=2; all QD/QW), dyspnea (n=2; both QD), and fatigue and pneumonia (each n=2; each 1 QD/QW, 1 QD). New-onset grade 3 thrombocytopenia was observed in 6/32 (19%) pts in QD/QW and 9/35 (26%) pts in QD; grade 4 thrombocytopenia was observed in 6/32 (19%) pts in QD/QW and 1/35 (3%) pts in QD. TEAEs of special interest included grade ≥2 diarrhea (n=4) and grade ≥2 rash (n=1) (all QD/QW). No colitis was reported. TEAEs led to parsaclisib interruption in 18/32 pts in QD/QW and 18/35 pts in QD, and rux interruption in 6/32 and 8/35 pts, respectively; and to parsaclisib discontinuation in 7/32 pts in QD/QW and 3/35 pts in QD, and rux discontinuation in 2/32 and 1/35 pts, respectively.

Conclusion
Add-on parsaclisib showed preliminary efficacy in pts with MF experiencing suboptimal response to rux; QD dosing appeared more efficacious than QD/QW dosing. Combination therapy was generally well tolerated with limited grade 3/4 AEs and TEAE-related discontinuations.
Keyword(s): Myelofibrosis, Phase II, PI3K, Ruxolitinib


