
Contributions
Abstract: EP1071
Type: E-Poster Presentation
Session title: Myeloproliferative neoplasms - Biology & Translational Research
Background
Mutations in CALR gene exon 9 insertion/deletions along with JAK2 or MPL gene mutations are routine diagnostic markers in phi-negative myeloproliferative neoplasms (MPN). Interferon-α (IFN) selectively targets the malignant clone in MPN patients and can induce both hematological and molecular remissions. Quantitative measurement of JAK2 (V617F) mutation burden is routinely used for treatment monitoring in the therapy of MPN. The value of CALR mutation burden monitoring still controversial.
Aims
To evaluate CALR gene mutation clearance in Russian patients with phi- negative MPNs, treated with IFN.
Methods
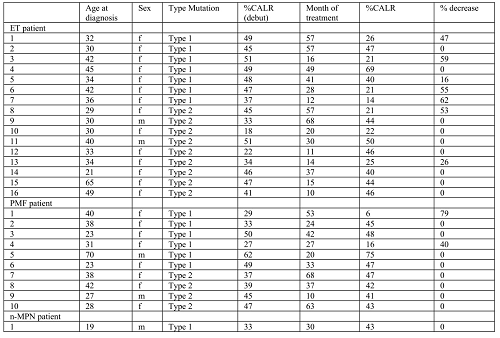
. Patients diagnosed in accordance with the WHO (2017) classification and treated at the Outpatient Department of the National Research Center for Hematology from 2015 to 2020 were included in the study (n=27). Among 27 patients with MPNs 16 cases were essential thrombocythemia (ET), 10 cases - primary myelofibrosis (PMF), 1 unclassified MPNs. All cases were tested for the presence of JAK2 (V617F), CALR(exon 9), and MPL (W515L/K) gene mutations by means of allele-specific PCR or fragment analysis. All patients were CALR positive (type 1 or type 2), JAK2 (V617F) and MPL (W515L/K) mutation negative at the onset of disease. Out of 27 patients, 22 were females (81%), and the mean age at diagnosis was 34.6 years (range 18-70). The median of IFN treatment duration was 30,5 months (range 10-68).
Results
Only 2 ET patients with CALR mutation type 2 achieved a minimal or partial molecular response. Both of them had at onset an initial allele load level of no more than 45%. Patients with PMF did not achieve any molecular response. 2 ET patients with the allelic load of 45 and 49% at the onset did not achieve any molecular response despite long-term treatment (49 months or more). Molecular response in patients with type 1 mutation was 7 out of 14 (50%) - 5 patients with ET and 2 – with PMF. Two PMF patients with molecular response to treatment had an allele load at onset of no more than 30%.
Conclusion
All patients in our study had more than 20% level of the mutant CALR allele at baseline, in contrast to patients with the JAK2 (V617F) mutation, who often carry low levels of mutant cells. Despite complete hematological remission achieved by all patients included in the study, clearance of mutated clone measured by PCR and fragment analysis of CALR mutation was only partial in some cases. One can speculate that IFN treatment may effectively control malignant cells with CALR mutations while not affecting more differentiated ones. However further studies with separated cell populations are required to support this point and to establish the value of CALR mutation monitoring in the therapy of MPNs.
Keyword(s): Interferon-alpha, Molecular response, Mutation analysis, Myeloproliferative disorder
Abstract: EP1071
Type: E-Poster Presentation
Session title: Myeloproliferative neoplasms - Biology & Translational Research
Background
Mutations in CALR gene exon 9 insertion/deletions along with JAK2 or MPL gene mutations are routine diagnostic markers in phi-negative myeloproliferative neoplasms (MPN). Interferon-α (IFN) selectively targets the malignant clone in MPN patients and can induce both hematological and molecular remissions. Quantitative measurement of JAK2 (V617F) mutation burden is routinely used for treatment monitoring in the therapy of MPN. The value of CALR mutation burden monitoring still controversial.
Aims
To evaluate CALR gene mutation clearance in Russian patients with phi- negative MPNs, treated with IFN.
Methods
. Patients diagnosed in accordance with the WHO (2017) classification and treated at the Outpatient Department of the National Research Center for Hematology from 2015 to 2020 were included in the study (n=27). Among 27 patients with MPNs 16 cases were essential thrombocythemia (ET), 10 cases - primary myelofibrosis (PMF), 1 unclassified MPNs. All cases were tested for the presence of JAK2 (V617F), CALR(exon 9), and MPL (W515L/K) gene mutations by means of allele-specific PCR or fragment analysis. All patients were CALR positive (type 1 or type 2), JAK2 (V617F) and MPL (W515L/K) mutation negative at the onset of disease. Out of 27 patients, 22 were females (81%), and the mean age at diagnosis was 34.6 years (range 18-70). The median of IFN treatment duration was 30,5 months (range 10-68).
Results
Only 2 ET patients with CALR mutation type 2 achieved a minimal or partial molecular response. Both of them had at onset an initial allele load level of no more than 45%. Patients with PMF did not achieve any molecular response. 2 ET patients with the allelic load of 45 and 49% at the onset did not achieve any molecular response despite long-term treatment (49 months or more). Molecular response in patients with type 1 mutation was 7 out of 14 (50%) - 5 patients with ET and 2 – with PMF. Two PMF patients with molecular response to treatment had an allele load at onset of no more than 30%.

Conclusion
All patients in our study had more than 20% level of the mutant CALR allele at baseline, in contrast to patients with the JAK2 (V617F) mutation, who often carry low levels of mutant cells. Despite complete hematological remission achieved by all patients included in the study, clearance of mutated clone measured by PCR and fragment analysis of CALR mutation was only partial in some cases. One can speculate that IFN treatment may effectively control malignant cells with CALR mutations while not affecting more differentiated ones. However further studies with separated cell populations are required to support this point and to establish the value of CALR mutation monitoring in the therapy of MPNs.
Keyword(s): Interferon-alpha, Molecular response, Mutation analysis, Myeloproliferative disorder


