
Contributions
Abstract: EP1067
Type: E-Poster Presentation
Session title: Myeloproliferative neoplasms - Biology & Translational Research
Background
Trained immunity (TI) is a pro-inflammatory program induced in monocyte/macrophages upon sensing of specific pathogens, and characterized by immunometabolic and epigenetic changes enhancing cytokine production. Maladaptive activation of TI (i.e. in the absence of infection) might result in detrimental inflammation and disease development.
Aims
The present study aims at identifying the role and extent of inappropriate activation of TI in the pathogenesis of a human inflammatory myeloid neoplasm (Erdheim-Chester disease, ECD, characterized by the BRAFV600E oncogenic mutation in monocyte/macrophages and excess cytokine production).
Methods
Human monocytes engineered to express BRAV600E were investigated for the ability to recapitulate TI and ECD features by combining biochemical, genetic, metabolic, and functional approaches.
Results
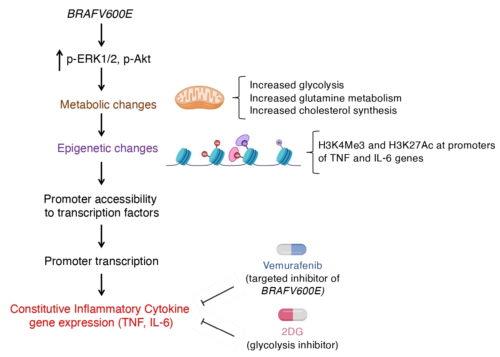
Mechanistically, we provide evidence that, myeloid cells expressing BRAFV600E exhibit all molecular features of TI: activation of the AKT/mTOR signaling axis; increased glycolysis, glutaminolysis, and cholesterol synthesis; epigenetic changes on promoters of genes encoding cytokines; and enhanced cytokine production leading to hyper-inflammatory responses. In ECD patients, effective therapeutic strategies contrast this maladaptive TI phenotype; in addition, pharmacologic inhibition of immunometabolic changes underlying TI (i.e. glycolysis) effectively dampens cytokine production by myeloid cells.
Conclusion
This study reveals the deleterious potential of inappropriate activation of TI in the pathogenesis of human inflammatory myeloid neoplasms, and the opportunity for inhibition of TI in conditions characterized by maladaptive myeloid-driven inflammation.
Keyword(s): Inflammation, Innate Immunity, Oncogene
Abstract: EP1067
Type: E-Poster Presentation
Session title: Myeloproliferative neoplasms - Biology & Translational Research
Background
Trained immunity (TI) is a pro-inflammatory program induced in monocyte/macrophages upon sensing of specific pathogens, and characterized by immunometabolic and epigenetic changes enhancing cytokine production. Maladaptive activation of TI (i.e. in the absence of infection) might result in detrimental inflammation and disease development.
Aims
The present study aims at identifying the role and extent of inappropriate activation of TI in the pathogenesis of a human inflammatory myeloid neoplasm (Erdheim-Chester disease, ECD, characterized by the BRAFV600E oncogenic mutation in monocyte/macrophages and excess cytokine production).
Methods
Human monocytes engineered to express BRAV600E were investigated for the ability to recapitulate TI and ECD features by combining biochemical, genetic, metabolic, and functional approaches.
Results
Mechanistically, we provide evidence that, myeloid cells expressing BRAFV600E exhibit all molecular features of TI: activation of the AKT/mTOR signaling axis; increased glycolysis, glutaminolysis, and cholesterol synthesis; epigenetic changes on promoters of genes encoding cytokines; and enhanced cytokine production leading to hyper-inflammatory responses. In ECD patients, effective therapeutic strategies contrast this maladaptive TI phenotype; in addition, pharmacologic inhibition of immunometabolic changes underlying TI (i.e. glycolysis) effectively dampens cytokine production by myeloid cells.

Conclusion
This study reveals the deleterious potential of inappropriate activation of TI in the pathogenesis of human inflammatory myeloid neoplasms, and the opportunity for inhibition of TI in conditions characterized by maladaptive myeloid-driven inflammation.
Keyword(s): Inflammation, Innate Immunity, Oncogene


