
Contributions
Abstract: EP1062
Type: E-Poster Presentation
Session title: Myeloproliferative neoplasms - Biology & Translational Research
Background
Myelofibrosis (MF) is a debilitating clonal myeloproliferative neoplasm characterized by anemia, splenomegaly, and constitutional symptoms, often presenting with activating Janus kinase 2 (JAK2) mutations. Two JAK inhibitors (JAKis), ruxolitinib (RUX) and fedratinib (FEDR), are approved for treatment (Tx) of intermediate- or high-risk MF, and others, such as momelotinib and pacritinib, are in late-stage clinical development. FEDR is an oral, selective inhibitor of wild-type and mutant JAK2 and fms related receptor tyrosine kinase 3 (FLT3) approved in the United States (US) and European Union (EU) for adult patients (pts) with intermediate-2 or high-risk primary or secondary MF. Ruxolitinib (RUX) is a multikinase inhibitor with broad activity against JAK family kinases including JAK1, JAK2, JAK3, and TYK2. RUX is approved in the US and EU for Tx of pts with intermediate or high-risk primary or secondary MF. Due to variations in JAK specificity, FEDR and RUX may have differential immunomodulation effects, which could have important clinical implications.
Aims
Determine the relative immunomodulatory activity of FEDR and RUX in human ex vivo culture model systems.
Methods
Peripheral blood mononuclear cells (PBMCs) were isolated from heathy subjects (n = 6) with standard Ficoll gradient centrifugation. Primary cells (PBMCs and CD56+ NK cells) and cell lines (Raji and K562) were co-cultured in triplicate with FEDR, RUX, or vehicle control at different effector:target (E:T) ratios and assayed by flow cytometry. Activation was assessed by ELISA for interferon γ (IFNγ) secretion after Tx with 50 ng/mL Staphylococcus enterotoxin B (SEB). Though the recommended clinical dose of FEDR, 400 mg/day, is ~10-fold higher than the recommended RUX dosages of 30–50 mg/day (15–25 mg BID), the observed peak plasma concentrations for both are ~1–2 µM. Thus, FEDR and RUX activity were compared by using equal concentrations of drug at 30, 100, 300, 600, 1000 nM, or vehicle control. A two-parameter, unpaired Student’s t-test was used to compare Tx groups.
Results
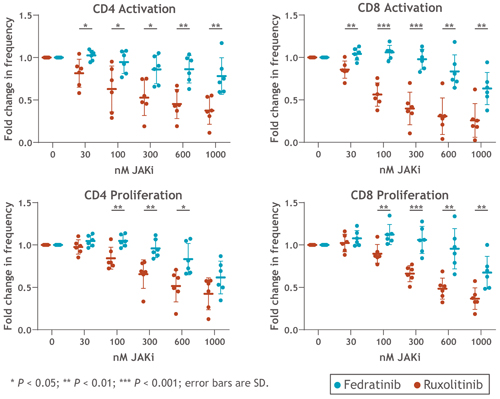
PBMCs were cultured with equal concentrations of FEDR or RUX. Overall, Tx with FEDR showed significantly reduced CD4+ and CD8+ T-cell suppression at clinically relevant doses compared with RUX. Compared with vehicle control, activation and proliferation of CD4+ T cells were suppressed with RUX Tx by 48% and 35%, respectively, and by 14% and 4% with FEDR Tx (each at 300 nM). Similarly, CD8+ T-cell activation was reduced by 60% and proliferation by 34% with RUX vs. control, compared with 2% and no reduction, with FEDR vs. control (P > 0.01) at 300 nM (Figure). In a co-culture system (E:T ratio, 3:1 NK:Raji cells) using 2 µg/mL rituximab (or isotype control) to tag CD20+ Raji cells, the reduction in NK-mediated antibody-dependent cellular cytotoxicity (ADCC) was significantly greater with RUX than FEDR after 4 hr. In assays of HLA-dependent cytotoxicity (E:T ratio, 20:1 NK:K562 cells; no antibody), FEDR showed limited inhibition of NK cells compared with RUX after 6 hr in 4/5 NK cell donor samples.
Conclusion
Overall, Tx with FEDR resulted in significantly lower suppression of T- and NK-cell proliferation and activation ex vivo when compared with RUX. FEDR also displayed limited suppression of NK-cell–mediated ADCC and HLA-dependent cytotoxicity in ex vivo model systems. These findings elucidate some of the variable immunomodulatory effects of JAKis and may aid in development of clinical trials combining FEDR with immunotherapies for MF and other neoplasms.
Keyword(s): Fedratinib, Immunomodulation, Myelofibrosis, Ruxolitinib
Abstract: EP1062
Type: E-Poster Presentation
Session title: Myeloproliferative neoplasms - Biology & Translational Research
Background
Myelofibrosis (MF) is a debilitating clonal myeloproliferative neoplasm characterized by anemia, splenomegaly, and constitutional symptoms, often presenting with activating Janus kinase 2 (JAK2) mutations. Two JAK inhibitors (JAKis), ruxolitinib (RUX) and fedratinib (FEDR), are approved for treatment (Tx) of intermediate- or high-risk MF, and others, such as momelotinib and pacritinib, are in late-stage clinical development. FEDR is an oral, selective inhibitor of wild-type and mutant JAK2 and fms related receptor tyrosine kinase 3 (FLT3) approved in the United States (US) and European Union (EU) for adult patients (pts) with intermediate-2 or high-risk primary or secondary MF. Ruxolitinib (RUX) is a multikinase inhibitor with broad activity against JAK family kinases including JAK1, JAK2, JAK3, and TYK2. RUX is approved in the US and EU for Tx of pts with intermediate or high-risk primary or secondary MF. Due to variations in JAK specificity, FEDR and RUX may have differential immunomodulation effects, which could have important clinical implications.
Aims
Determine the relative immunomodulatory activity of FEDR and RUX in human ex vivo culture model systems.
Methods
Peripheral blood mononuclear cells (PBMCs) were isolated from heathy subjects (n = 6) with standard Ficoll gradient centrifugation. Primary cells (PBMCs and CD56+ NK cells) and cell lines (Raji and K562) were co-cultured in triplicate with FEDR, RUX, or vehicle control at different effector:target (E:T) ratios and assayed by flow cytometry. Activation was assessed by ELISA for interferon γ (IFNγ) secretion after Tx with 50 ng/mL Staphylococcus enterotoxin B (SEB). Though the recommended clinical dose of FEDR, 400 mg/day, is ~10-fold higher than the recommended RUX dosages of 30–50 mg/day (15–25 mg BID), the observed peak plasma concentrations for both are ~1–2 µM. Thus, FEDR and RUX activity were compared by using equal concentrations of drug at 30, 100, 300, 600, 1000 nM, or vehicle control. A two-parameter, unpaired Student’s t-test was used to compare Tx groups.
Results
PBMCs were cultured with equal concentrations of FEDR or RUX. Overall, Tx with FEDR showed significantly reduced CD4+ and CD8+ T-cell suppression at clinically relevant doses compared with RUX. Compared with vehicle control, activation and proliferation of CD4+ T cells were suppressed with RUX Tx by 48% and 35%, respectively, and by 14% and 4% with FEDR Tx (each at 300 nM). Similarly, CD8+ T-cell activation was reduced by 60% and proliferation by 34% with RUX vs. control, compared with 2% and no reduction, with FEDR vs. control (P > 0.01) at 300 nM (Figure). In a co-culture system (E:T ratio, 3:1 NK:Raji cells) using 2 µg/mL rituximab (or isotype control) to tag CD20+ Raji cells, the reduction in NK-mediated antibody-dependent cellular cytotoxicity (ADCC) was significantly greater with RUX than FEDR after 4 hr. In assays of HLA-dependent cytotoxicity (E:T ratio, 20:1 NK:K562 cells; no antibody), FEDR showed limited inhibition of NK cells compared with RUX after 6 hr in 4/5 NK cell donor samples.

Conclusion
Overall, Tx with FEDR resulted in significantly lower suppression of T- and NK-cell proliferation and activation ex vivo when compared with RUX. FEDR also displayed limited suppression of NK-cell–mediated ADCC and HLA-dependent cytotoxicity in ex vivo model systems. These findings elucidate some of the variable immunomodulatory effects of JAKis and may aid in development of clinical trials combining FEDR with immunotherapies for MF and other neoplasms.
Keyword(s): Fedratinib, Immunomodulation, Myelofibrosis, Ruxolitinib


