
Contributions
Abstract: EP1058
Type: E-Poster Presentation
Session title: Myeloproliferative neoplasms - Biology & Translational Research
Background
Myeloproliferative neoplasms (MPN) most frequently originate from mutations in hematopoietic stem and progenitor cells (HSPCs) affecting JAK2 or CALR. Tamoxifen restores normal apoptosis in mutated HSPCs in preclinical studies. The results of the TAMARIN Phase II study assessing tamoxifen's safety and activity in reducing molecular markers of disease burden in MPN have been recently reported (Blood 2020;136(Supplement 1):33-35).
Aims
Here we have investigated tamoxifen’s mechanism of action in human MPN.
Methods
CD34+ HSPCs were isolated from peripheral blood at baseline, 12w and 24w after tamoxifen treatment for RNA-Seq, also performed on JAK2V617F-mutated human cell lines 4h after 4-hydroxytamoxifen (4OH-TAM) treatment. ATP generation from Oxidative phosphorylation (OXPHOS) and glycolysis was measured by Seahorse. STAT5-Y694 phosphorylation and apoptosis were measured by flow cytometry in Ba/F3 cells expressing erythropoietin receptor (EpoR) and WT or mutant JAK2, HEL, SET2 and UKE1 cells treated with different drugs including unfolded protein response (UPR) stressors thapsigargin and tunicamycin.
Results
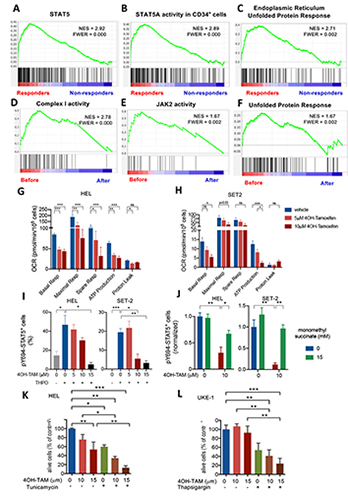
Weighted correlation network analysis (WGCNA) revealed two gene modules with increased expression in HSPCs from responders at baseline. Gene-set enrichment analysis (GSEA) highlighted in responder HSPCs genes related to STAT3, STAT5, inflammation, apoptosis, reactive oxygen species (ROS), starvation or ATF response to UPR and cellular stress, suggesting that differential UPR determines sensitivity to tamoxifen. Indeed, nutrient deprivation or UPR stressors sensitised JAK2V617F+ cells to 4OH-TAM-induced cell death. 4OH-TAM-induced cell death was more pronounced in Ba/F3 cells carrying the mutant human JAK2V617F, supporting an increased activity of tamoxifen in the mutant cells. The expression of OXPHOS-related genes was high at baseline and specifically reduced by tamoxifen in responder HSPCs. This was mimicked by 4OH-TAM-sensitive and resistant cell lines. OXPHOS-derived ATP was markedly reduced by 4OH-TAM in sensitive cells including permeabilised cells, suggesting a direct inhibition of mitochondrial complex I. Complex II activators (succinate prodrug) protected from 4OH-TAM-induced cell death by rescuing ATP generation. Since ATP binding to the pseudokinase domain of JAK2 is critical for pathogenic activation of JAK2V617F, but is not required for WT JAK2 activity, we hypothesized that reduced ATP generation might compromise pathogenic JAK2V617F-STAT5 activation and possibly explain the selective proapoptotic effect of tamoxifen in mutant cells. STAT5-Y694 phosphorylation after Epo or Tpo stimulation was dose-dependently inhibited by 4OH-TAM. However, restoration of the mitochondrial electron transport and ATP generation by succinate prodrugs or cell-permeable ATP rescued pathogenic JAK2V617F-STAT5 activation in 4OH-TAM-treated cells.
Conclusion
Longitudinal transcriptomic analysis of HSPCs from TAMARIN study patients and JAK2V617F-mutated cells lines showed high UPR activity and expression of OXPHOS genes, specifically reduced by tamoxifen in sensitive cells. 4OH-TAM inhibits mitochondrial complex I and ensuing ATP synthesis, causing decreased pathogenic (but not WT) JAK2 signalling. These studies advocate for future studies on the transcriptomic and metabolic effects of selective oestrogen receptor modulators to target metabolic vulnerabilities in MPN with careful consideration of thrombotic risk.
Keyword(s): Apoptosis, Clinical trial, Mitochondria, Myeloproliferative disorder
Abstract: EP1058
Type: E-Poster Presentation
Session title: Myeloproliferative neoplasms - Biology & Translational Research
Background
Myeloproliferative neoplasms (MPN) most frequently originate from mutations in hematopoietic stem and progenitor cells (HSPCs) affecting JAK2 or CALR. Tamoxifen restores normal apoptosis in mutated HSPCs in preclinical studies. The results of the TAMARIN Phase II study assessing tamoxifen's safety and activity in reducing molecular markers of disease burden in MPN have been recently reported (Blood 2020;136(Supplement 1):33-35).
Aims
Here we have investigated tamoxifen’s mechanism of action in human MPN.
Methods
CD34+ HSPCs were isolated from peripheral blood at baseline, 12w and 24w after tamoxifen treatment for RNA-Seq, also performed on JAK2V617F-mutated human cell lines 4h after 4-hydroxytamoxifen (4OH-TAM) treatment. ATP generation from Oxidative phosphorylation (OXPHOS) and glycolysis was measured by Seahorse. STAT5-Y694 phosphorylation and apoptosis were measured by flow cytometry in Ba/F3 cells expressing erythropoietin receptor (EpoR) and WT or mutant JAK2, HEL, SET2 and UKE1 cells treated with different drugs including unfolded protein response (UPR) stressors thapsigargin and tunicamycin.
Results
Weighted correlation network analysis (WGCNA) revealed two gene modules with increased expression in HSPCs from responders at baseline. Gene-set enrichment analysis (GSEA) highlighted in responder HSPCs genes related to STAT3, STAT5, inflammation, apoptosis, reactive oxygen species (ROS), starvation or ATF response to UPR and cellular stress, suggesting that differential UPR determines sensitivity to tamoxifen. Indeed, nutrient deprivation or UPR stressors sensitised JAK2V617F+ cells to 4OH-TAM-induced cell death. 4OH-TAM-induced cell death was more pronounced in Ba/F3 cells carrying the mutant human JAK2V617F, supporting an increased activity of tamoxifen in the mutant cells. The expression of OXPHOS-related genes was high at baseline and specifically reduced by tamoxifen in responder HSPCs. This was mimicked by 4OH-TAM-sensitive and resistant cell lines. OXPHOS-derived ATP was markedly reduced by 4OH-TAM in sensitive cells including permeabilised cells, suggesting a direct inhibition of mitochondrial complex I. Complex II activators (succinate prodrug) protected from 4OH-TAM-induced cell death by rescuing ATP generation. Since ATP binding to the pseudokinase domain of JAK2 is critical for pathogenic activation of JAK2V617F, but is not required for WT JAK2 activity, we hypothesized that reduced ATP generation might compromise pathogenic JAK2V617F-STAT5 activation and possibly explain the selective proapoptotic effect of tamoxifen in mutant cells. STAT5-Y694 phosphorylation after Epo or Tpo stimulation was dose-dependently inhibited by 4OH-TAM. However, restoration of the mitochondrial electron transport and ATP generation by succinate prodrugs or cell-permeable ATP rescued pathogenic JAK2V617F-STAT5 activation in 4OH-TAM-treated cells.

Conclusion
Longitudinal transcriptomic analysis of HSPCs from TAMARIN study patients and JAK2V617F-mutated cells lines showed high UPR activity and expression of OXPHOS genes, specifically reduced by tamoxifen in sensitive cells. 4OH-TAM inhibits mitochondrial complex I and ensuing ATP synthesis, causing decreased pathogenic (but not WT) JAK2 signalling. These studies advocate for future studies on the transcriptomic and metabolic effects of selective oestrogen receptor modulators to target metabolic vulnerabilities in MPN with careful consideration of thrombotic risk.
Keyword(s): Apoptosis, Clinical trial, Mitochondria, Myeloproliferative disorder


