
Contributions
Abstract: EP1050
Type: E-Poster Presentation
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
Maintenance therapy deepens response and prolongs progression free survival (PFS) in patients with newly diagnosed multiple myeloma (NDMM) after frontline regimens. Ixazomib, a 2nd generation oral proteasome inhibitor (PI), has been approved for maintenance therapy because of the convenience and tolerability.
Aims
We conducted this prospective multi-center study to compare the efficacy and safety of Ixazomib (I-MT) or Ixazomib plus Lenalidomide (IL-MT) to Lenalidomide (L-MT) as maintenance regimen in NDMM patients.
Methods
This study was approved by the Institutional Review Board of Peking Union Medical College Hospital and registered (NCT04217967). NDMM patients were enrolled from 7 centers of North China MM Registry, since September 2019. After 4 cycles of front-line induction therapy, patients reached partial response (PR) or above would receive autologous stem cell transplantation (ASCT) if eligible, or another up to 5 cycles of same regimens if ineligible, then started maintenance. Patients who did not achieve PR within 4 cycles would switch to second-line induction for 2-5 cycles and start maintenance once PR or above was achieved. For maintenance therapy, Ixazomib was given 4mg on day 1,8,15, and 25mg every other day for Lenalidomide on days 1–21 of 28day cycles. Patients in dual drug group were administrated with both Ixazomib and Lenalidomide. The primary endpoint was PFS from maintenance.
Results
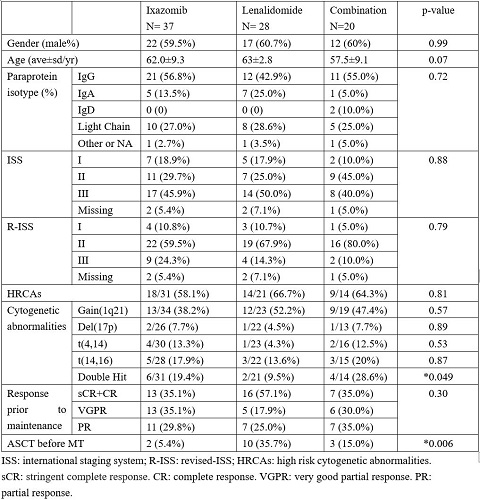
A total of 85 patients were enrolled, including 37 in I-MT, 28 in L-MT and 20 in IL-MT. The demographic and clinical characteristics were comparable among three groups at baseline, including gender ratio, age, paraprotein isotype, ISS, R-ISS, and response evaluation before MT (Figure 1). Though patients on IL-MT were slightly younger. The proportions of patients with high-risk cytogenetic abnormalities (HRCAs), defined as amplification 1q21 (1q21+), deletion 17p (17p-), t(4,14) and t(14,16), were also comparable, as well as the ratios of each CA. However, L-MT cohort had a lower percentage of patients with double hit CA. The ratio of ASCT in L-MT cohort was also greater the others.
The median follow-up duration since maintenance was 4.0, 8.5 and 4.6 months in I-MT, L-MT and IL-MT groups, respectively. Disease progression was recorded in 2 patients (5.4%) on I-MT, 3 (10.7%) on L-MT and 2 (7.1%) on IL-MT. The median PFS was not reached (NR) in all groups. Meanwhile, 6 patients (16.2%) on I, 2 (7.1%) on L and 6 (30%) on IL had improved response after maintenance. No mortality was recorded in all patients.
Peripheral neuropathy with grade 1 was observed in 4 patients (10.8%) on I-MT, 7 (35%) on IL-MT and 0 on L-MT. Gastrointestinal events occurred in 5 patients (13.5%) on I-MT, 5 (25%) on IL-MT and 0 on L-MT. The prevalence of hematologic toxicities was 2.7%, 10% and 7.1%, respectively. Whereas infection rate was 8.1%, 5% and 3.6%. The incidence of skin rashes was 2.7%, 5% and 10.7%, respectively. No patients have discontinued maintenance due to adverse events.
Conclusion
Due to inadequate access to melphalan and low rate of ASCT in China, there is still a gap of PFS in NDMM patients with those in western countries. We herein design this multi-centered prospective study to evaluate if dual drug maintenance will further strengthen response and make up the gap. Though the primary endpoint--PFS has not been reached in all treatment groups, dual drug maintenance is quite tolerable. Clinicians prefer to administrate Ixazomib in patients with complex cytogenetic abnormalities in the real-world practice.
Keyword(s): Maintenance, Multiple myeloma
Abstract: EP1050
Type: E-Poster Presentation
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
Maintenance therapy deepens response and prolongs progression free survival (PFS) in patients with newly diagnosed multiple myeloma (NDMM) after frontline regimens. Ixazomib, a 2nd generation oral proteasome inhibitor (PI), has been approved for maintenance therapy because of the convenience and tolerability.
Aims
We conducted this prospective multi-center study to compare the efficacy and safety of Ixazomib (I-MT) or Ixazomib plus Lenalidomide (IL-MT) to Lenalidomide (L-MT) as maintenance regimen in NDMM patients.
Methods
This study was approved by the Institutional Review Board of Peking Union Medical College Hospital and registered (NCT04217967). NDMM patients were enrolled from 7 centers of North China MM Registry, since September 2019. After 4 cycles of front-line induction therapy, patients reached partial response (PR) or above would receive autologous stem cell transplantation (ASCT) if eligible, or another up to 5 cycles of same regimens if ineligible, then started maintenance. Patients who did not achieve PR within 4 cycles would switch to second-line induction for 2-5 cycles and start maintenance once PR or above was achieved. For maintenance therapy, Ixazomib was given 4mg on day 1,8,15, and 25mg every other day for Lenalidomide on days 1–21 of 28day cycles. Patients in dual drug group were administrated with both Ixazomib and Lenalidomide. The primary endpoint was PFS from maintenance.
Results
A total of 85 patients were enrolled, including 37 in I-MT, 28 in L-MT and 20 in IL-MT. The demographic and clinical characteristics were comparable among three groups at baseline, including gender ratio, age, paraprotein isotype, ISS, R-ISS, and response evaluation before MT (Figure 1). Though patients on IL-MT were slightly younger. The proportions of patients with high-risk cytogenetic abnormalities (HRCAs), defined as amplification 1q21 (1q21+), deletion 17p (17p-), t(4,14) and t(14,16), were also comparable, as well as the ratios of each CA. However, L-MT cohort had a lower percentage of patients with double hit CA. The ratio of ASCT in L-MT cohort was also greater the others.
The median follow-up duration since maintenance was 4.0, 8.5 and 4.6 months in I-MT, L-MT and IL-MT groups, respectively. Disease progression was recorded in 2 patients (5.4%) on I-MT, 3 (10.7%) on L-MT and 2 (7.1%) on IL-MT. The median PFS was not reached (NR) in all groups. Meanwhile, 6 patients (16.2%) on I, 2 (7.1%) on L and 6 (30%) on IL had improved response after maintenance. No mortality was recorded in all patients.
Peripheral neuropathy with grade 1 was observed in 4 patients (10.8%) on I-MT, 7 (35%) on IL-MT and 0 on L-MT. Gastrointestinal events occurred in 5 patients (13.5%) on I-MT, 5 (25%) on IL-MT and 0 on L-MT. The prevalence of hematologic toxicities was 2.7%, 10% and 7.1%, respectively. Whereas infection rate was 8.1%, 5% and 3.6%. The incidence of skin rashes was 2.7%, 5% and 10.7%, respectively. No patients have discontinued maintenance due to adverse events.

Conclusion
Due to inadequate access to melphalan and low rate of ASCT in China, there is still a gap of PFS in NDMM patients with those in western countries. We herein design this multi-centered prospective study to evaluate if dual drug maintenance will further strengthen response and make up the gap. Though the primary endpoint--PFS has not been reached in all treatment groups, dual drug maintenance is quite tolerable. Clinicians prefer to administrate Ixazomib in patients with complex cytogenetic abnormalities in the real-world practice.
Keyword(s): Maintenance, Multiple myeloma


