
Contributions
Abstract: EP1039
Type: E-Poster Presentation
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
Over the past two decades, the approval of several novel drugs has broadened therapeutic options for multiple myeloma (MM) patients, but has also raised concerns about increasing drug expenses and healthcare costs in general. Access to these treatments in public and private settings in Brazil differs significantly and it is unknown if shortcomings in treatment availability affect patient outcomes.
Aims
To understand the patient characteristics, treatment patterns and MM patient outcomes in private and public settings in Brazil.
Methods
MMyBrave is an observational, retrospective study with no control group . Eligible patients were diagnosed with active MM between January 1, 2008 and December 31, 2016 (cut-off date), at 17 public and private medical centers in Brazil. The MM diagnosis was performed at the discretion of the investigator. The data collection started on June 2018, after ethical approval, and progressed until August 2019. Sites with patients from both private and public sectors were considered hybrid centers.
Results
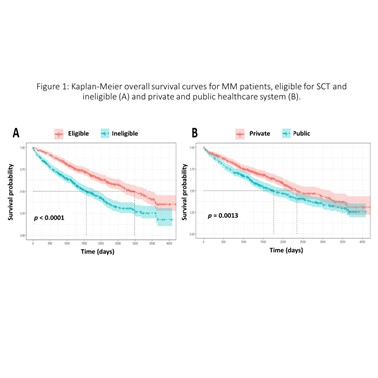
A total of 943 patients were included. Private, public and hybrid centers accounted for 49.3, 43.7 and 7.0% of patients, respectively. The mean age and male percentage at private, public and hybrid centers were 68.6, 68.5 and 66.0 years and 56.3, 51.2 and 50.0%, respectively. The International Staging System stages I, II and III and unknown stages were: 24.7, 24.1, 26.7 and 24.5 at private centers; 14.3, 18.4, 24.3 and 43.0 at public centers, and; 12.1, 19.7, 40.9 and 27.3% at hybrid centers. A total of 47.9% (41.8% public, 50.4% private and 7.7% hybrid) were eligible for stem cell transplantation (SCT). Proteasome inhibitor (PI) regimens (mainly regimens with bortezomib) were used by 50% at private centers (56% in SCT eligible (TE); and 44% in SCT ineligible (TI)), 11% at public centers (46% TE and 54% TI) and 8% at hybrid centers (80% TE and 20% TI). Regarding immunomodulator regimens (mainly thalidomide and rarely lenalidomide), 39% at private centers (53% TE and 47% TI), 71% at public centers (53% TE and 47% TI) and 80% at hybrid centers (58% TE and 42% TI). PI-immunomodulator regimens were used by 4% at private centers (93% TE and 7% TI) and 2% at public centres (17% TE and 83% TI). During the second-line treatment, PI regimens accounted for 46% at private centers, 31% at public centers and 17% at hybrid centers; immunomodulator regimens accounted for 27% at private centers (96% thalidomide and 4% lenalidomide), 57% at public centers (99% thalidomide and 1% lenalidomide) and 61% at hybrid centers (100% thalidomide). PI-immunomodulator regimens accounted for 16% at private centers and 3.0% at public centers. Less than 1% of patients have been treated with carfilzomib, daratumumab, ixazomib and pomalidomide. The median OS was 6.4 and 4.8 years (p = 0.001) for patients in the private and public systems; 8.2 and 4.3 years for TE (intention-to-treat population) and TI (p < 0.0001), respectively.
Conclusion
Real-world MM patient outcomes at private and public institutions in Brazil were quite different. The superior OS among patients in the private healthcare system may be due, at least in part, to a wider availability of therapies enabling individualized management of MM patients.
Keyword(s): Health care, Multiple myeloma, Proteasome inhibitor, Treatment
Abstract: EP1039
Type: E-Poster Presentation
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
Over the past two decades, the approval of several novel drugs has broadened therapeutic options for multiple myeloma (MM) patients, but has also raised concerns about increasing drug expenses and healthcare costs in general. Access to these treatments in public and private settings in Brazil differs significantly and it is unknown if shortcomings in treatment availability affect patient outcomes.
Aims
To understand the patient characteristics, treatment patterns and MM patient outcomes in private and public settings in Brazil.
Methods
MMyBrave is an observational, retrospective study with no control group . Eligible patients were diagnosed with active MM between January 1, 2008 and December 31, 2016 (cut-off date), at 17 public and private medical centers in Brazil. The MM diagnosis was performed at the discretion of the investigator. The data collection started on June 2018, after ethical approval, and progressed until August 2019. Sites with patients from both private and public sectors were considered hybrid centers.
Results
A total of 943 patients were included. Private, public and hybrid centers accounted for 49.3, 43.7 and 7.0% of patients, respectively. The mean age and male percentage at private, public and hybrid centers were 68.6, 68.5 and 66.0 years and 56.3, 51.2 and 50.0%, respectively. The International Staging System stages I, II and III and unknown stages were: 24.7, 24.1, 26.7 and 24.5 at private centers; 14.3, 18.4, 24.3 and 43.0 at public centers, and; 12.1, 19.7, 40.9 and 27.3% at hybrid centers. A total of 47.9% (41.8% public, 50.4% private and 7.7% hybrid) were eligible for stem cell transplantation (SCT). Proteasome inhibitor (PI) regimens (mainly regimens with bortezomib) were used by 50% at private centers (56% in SCT eligible (TE); and 44% in SCT ineligible (TI)), 11% at public centers (46% TE and 54% TI) and 8% at hybrid centers (80% TE and 20% TI). Regarding immunomodulator regimens (mainly thalidomide and rarely lenalidomide), 39% at private centers (53% TE and 47% TI), 71% at public centers (53% TE and 47% TI) and 80% at hybrid centers (58% TE and 42% TI). PI-immunomodulator regimens were used by 4% at private centers (93% TE and 7% TI) and 2% at public centres (17% TE and 83% TI). During the second-line treatment, PI regimens accounted for 46% at private centers, 31% at public centers and 17% at hybrid centers; immunomodulator regimens accounted for 27% at private centers (96% thalidomide and 4% lenalidomide), 57% at public centers (99% thalidomide and 1% lenalidomide) and 61% at hybrid centers (100% thalidomide). PI-immunomodulator regimens accounted for 16% at private centers and 3.0% at public centers. Less than 1% of patients have been treated with carfilzomib, daratumumab, ixazomib and pomalidomide. The median OS was 6.4 and 4.8 years (p = 0.001) for patients in the private and public systems; 8.2 and 4.3 years for TE (intention-to-treat population) and TI (p < 0.0001), respectively.

Conclusion
Real-world MM patient outcomes at private and public institutions in Brazil were quite different. The superior OS among patients in the private healthcare system may be due, at least in part, to a wider availability of therapies enabling individualized management of MM patients.
Keyword(s): Health care, Multiple myeloma, Proteasome inhibitor, Treatment


