
Contributions
Abstract: EP1038
Type: E-Poster Presentation
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
Carfilzomib is approved for relapsed multiple myeloma (RRMM) based on 2 randomized controlled trials (RCTs). The ASPIRE trial of KRd after 1-3 prior lines (PLs) of therapy showed a progression-free survival (PFS) of 26.3 and overall survival (OS) of 48.3 mo,1 while the ENDEAVOR trial of Kd reported a PFS of 18.7 and OS of 47.6 mo.2 A recent analysis showed that >75% of patients (pts) with RRMM in routine practice would have been ineligible for pivotal RCTs.3 Therefore, evaluation of the real-world (RW) efficacy of these regimens is needed.4
Aims
To evaluate the efficacy of carfilzomib-based regimens in a RW data set of pts with RRMM and 1-3 PLs of therapy.
Methods
We performed a retrospective study using the Canadian Myeloma Research Group Database (CMRG-DB), which includes >7500 MM pts from 15 Canadian sites and legacy data from 2007. All RRMM pts treated with approved carfilzomib-based therapies after 1-3 PLs analyzed up to 31/10/2020 were included. Primary outcome was PFS with each therapy; overall response rate (ORR), ≥very good partial response rate (VGPR), and OS were secondary outcomes. Survival was estimated using Kaplan-Meier methods.
Results
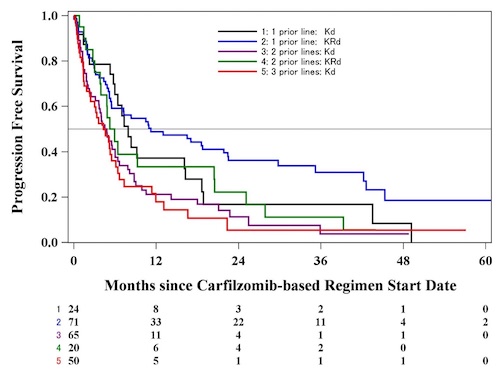
A total of 235 pts were identified: 139 treated with Kd and 96 with KRd. In the Kd group, 131 (94.2%) had prior PI and 93 (66.9%) had prior IMiD. For the KRd group, 93 (96.9%) had prior PI, and 19 (19.8%) had prior IMiD. In the Kd cohort, 24 (17.3%) of pts had 1 PL, 65 (46.8%) had two PLs, and 50 (36.0%) had three PLs of therapy. In the KRd group, 71 (74%), 20 (20.8%) and 5 (5.2%) had one, two, or three PLs, respectively.
The ORR of the entire Kd cohort was 49.6%, with ≥VGPR in 27.0%. Median PFS was 5.2 mo and OS was 16.7 mo. By PL of treatment in the Kd cohort: ORR was 69.6%, ≥VGPR was 30.4%, median PFS was 7.92 mo and OS was 34.0 mo for pts with 1 PL; ORR was 48.1%, ≥VGPR was 28.9%, median PFS was 4.80 mo and OS was 19.69 mo for pts with two PLs; and ORR was 38.9%, ≥ VGPR was 22.2%, median PFS was 4.31 mo, and OS was 12.39 mo for pts with three PLs.
The ORR of the entire KRd cohort was 67.1%, with 53.2% of pts achieving ≥VGPR. The median PFS was 10.49 mo and OS was 42.84 mo. For 1PL, ORR was 66.1%, ≥VGPR was 50%, PFS was 11.21 mo, and OS was 42.84 mo; for 2PLs, ORR was 54.6%, ≥VGPR was 27.3%, median PFS was 5.61 mo, and OS was 15.52 mo. The 5 pts with three PLs were too few to evaluate separately.
Conclusion
In this real-world observational study, the responses and efficacy were less than that demonstrated in prospective RCTs. However, our findings are consistent with those reported by Chari et al in 208 patients with relapsed MM treated with KRd after a median of 2 PLs who experienced a median TTNT of 9.5 months5 and in other real-world observational studies of carfilzomib-based regimens.6,7 An improved understanding of the reasons underlying the difference in effectiveness, such as recent availability of more potent initial treatments, may lead to improved clinical decision-making and translate into better real-world outcomes.
1. Stewart et al, NEJM 2015 2. Dimopoulos et al, Lancet Oncol 2017 3. Chari et al, Clin Lymph Myel Leuk 2020 4. Richardson et al, Blood Cancer J 2018 5. Chari et al, Expert Rev Hematol 2020 6. Durie et al, Haematologica 2017 7. Danhof et al, Clin Lymph Myel Leuk 2015
Keyword(s): Multiple myeloma, Relapse, Treatment
Abstract: EP1038
Type: E-Poster Presentation
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
Carfilzomib is approved for relapsed multiple myeloma (RRMM) based on 2 randomized controlled trials (RCTs). The ASPIRE trial of KRd after 1-3 prior lines (PLs) of therapy showed a progression-free survival (PFS) of 26.3 and overall survival (OS) of 48.3 mo,1 while the ENDEAVOR trial of Kd reported a PFS of 18.7 and OS of 47.6 mo.2 A recent analysis showed that >75% of patients (pts) with RRMM in routine practice would have been ineligible for pivotal RCTs.3 Therefore, evaluation of the real-world (RW) efficacy of these regimens is needed.4
Aims
To evaluate the efficacy of carfilzomib-based regimens in a RW data set of pts with RRMM and 1-3 PLs of therapy.
Methods
We performed a retrospective study using the Canadian Myeloma Research Group Database (CMRG-DB), which includes >7500 MM pts from 15 Canadian sites and legacy data from 2007. All RRMM pts treated with approved carfilzomib-based therapies after 1-3 PLs analyzed up to 31/10/2020 were included. Primary outcome was PFS with each therapy; overall response rate (ORR), ≥very good partial response rate (VGPR), and OS were secondary outcomes. Survival was estimated using Kaplan-Meier methods.
Results
A total of 235 pts were identified: 139 treated with Kd and 96 with KRd. In the Kd group, 131 (94.2%) had prior PI and 93 (66.9%) had prior IMiD. For the KRd group, 93 (96.9%) had prior PI, and 19 (19.8%) had prior IMiD. In the Kd cohort, 24 (17.3%) of pts had 1 PL, 65 (46.8%) had two PLs, and 50 (36.0%) had three PLs of therapy. In the KRd group, 71 (74%), 20 (20.8%) and 5 (5.2%) had one, two, or three PLs, respectively.
The ORR of the entire Kd cohort was 49.6%, with ≥VGPR in 27.0%. Median PFS was 5.2 mo and OS was 16.7 mo. By PL of treatment in the Kd cohort: ORR was 69.6%, ≥VGPR was 30.4%, median PFS was 7.92 mo and OS was 34.0 mo for pts with 1 PL; ORR was 48.1%, ≥VGPR was 28.9%, median PFS was 4.80 mo and OS was 19.69 mo for pts with two PLs; and ORR was 38.9%, ≥ VGPR was 22.2%, median PFS was 4.31 mo, and OS was 12.39 mo for pts with three PLs.
The ORR of the entire KRd cohort was 67.1%, with 53.2% of pts achieving ≥VGPR. The median PFS was 10.49 mo and OS was 42.84 mo. For 1PL, ORR was 66.1%, ≥VGPR was 50%, PFS was 11.21 mo, and OS was 42.84 mo; for 2PLs, ORR was 54.6%, ≥VGPR was 27.3%, median PFS was 5.61 mo, and OS was 15.52 mo. The 5 pts with three PLs were too few to evaluate separately.

Conclusion
In this real-world observational study, the responses and efficacy were less than that demonstrated in prospective RCTs. However, our findings are consistent with those reported by Chari et al in 208 patients with relapsed MM treated with KRd after a median of 2 PLs who experienced a median TTNT of 9.5 months5 and in other real-world observational studies of carfilzomib-based regimens.6,7 An improved understanding of the reasons underlying the difference in effectiveness, such as recent availability of more potent initial treatments, may lead to improved clinical decision-making and translate into better real-world outcomes.
1. Stewart et al, NEJM 2015 2. Dimopoulos et al, Lancet Oncol 2017 3. Chari et al, Clin Lymph Myel Leuk 2020 4. Richardson et al, Blood Cancer J 2018 5. Chari et al, Expert Rev Hematol 2020 6. Durie et al, Haematologica 2017 7. Danhof et al, Clin Lymph Myel Leuk 2015
Keyword(s): Multiple myeloma, Relapse, Treatment


