Contributions
Abstract: EP1033
Type: E-Poster Presentation
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
Several recent clinical trials in relapsed refractory multiple myeloma (RRMM) have demonstrated significant improvements in overall response rates (ORR), complete response (CR), and progression-free survival (PFS); however, few have reported improvement in overall survival (OS). As the “gold standard” for measuring the clinical benefits of a cancer drug, OS requires larger patient cohorts and longer follow-ups compared with other clinical trial endpoints.
Aims
The surrogacy of PFS for OS has been previously reported in RRMM; however, these analyses did not include most recent studies. This analysis aimed to investigate ORR and CR as potential surrogate endpoints for OS.
Methods
A systematic literature review of randomized controlled trials (RCTs) and single-arm studies in RRMM was conducted to identify studies that reported ORR or CR in addition to median OS. Medline, Embase, and Cochrane databases were systematically searched for randomized controlled trials published from 2010 to Jul 2020. Relevant congress abstracts from 2018 to 2020 were also reviewed. Correlations of OS with ORR and with CR were evaluated using Pearson’s product–moment correlation weighted by study sample size. Unadjusted and adjusted weighted linear regression models were constructed to estimate the gain in median OS associated with each surrogate endpoint. Optimal adjusted models considered age, sex, study year, MM type, International Staging System risk stage, prior stem cell transplant, prior lines of therapy, and treatment type.
Results
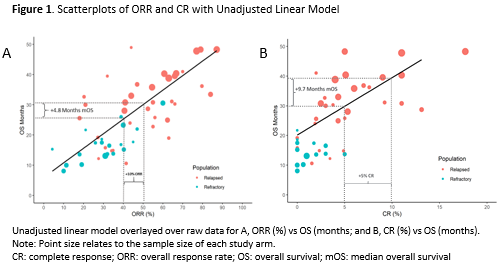
Sixty-five studies were identified, including 55 treatment arms from 33 RCTs. Moderate-to-strong significant correlations were observed for median OS with ORR (Pearson r=0.79) and CR (r=0.57). The unadjusted absolute analysis models estimated an increase of 0.48 (95% CI: 0.39, 0.57) and 1.94 (95% CI: 1.36, 2.51) months of median OS on average for every additional percentage point of ORR and CR, respectively (Figure 1). Following adjustment for age, relapsed vs refractory MM, and study year, the association was essentially unchanged at 0.46 (95% CI: 0.34, 0.58) and attenuated to 1.17 (95% CI: 0.65, 1.69) months of median OS for each additional point of ORR and CR, respectively.
Conclusion
We demonstrated significant associations of median OS with ORR and CR, supporting the use of ORR and CR as surrogate endpoints for OS benefit in RRMM. Because MM is considered incurable, increasing survival is a key endpoint from both a clinical standpoint and a humanistic standpoint. Being able to predict survival outcomes through endpoints available earlier in a trial may help expedite the approval of novel therapeutics to address this unmet need. In addition, based on unadjusted analysis, there is a clear trend towards higher ORR and CR rates and longer survival among patients with relapsed disease compared with patients with refractory disease. Adjusted models estimated that a 10% increase in ORR predicts a 4.6 month increase in median OS, and a 5% increase in CR predicts a 5.85 month increase in median OS. Often, early phase trials provide initial evidence of treatment benefit, but the follow-up is not long enough to calculate OS. These results suggest that ORR results in initial trials can be used to project survival outcomes, thus potentially informing the design of subsequent trials and accelerating patient care and treatment.
Keyword(s): Multiple myeloma, Survival
Abstract: EP1033
Type: E-Poster Presentation
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
Several recent clinical trials in relapsed refractory multiple myeloma (RRMM) have demonstrated significant improvements in overall response rates (ORR), complete response (CR), and progression-free survival (PFS); however, few have reported improvement in overall survival (OS). As the “gold standard” for measuring the clinical benefits of a cancer drug, OS requires larger patient cohorts and longer follow-ups compared with other clinical trial endpoints.
Aims
The surrogacy of PFS for OS has been previously reported in RRMM; however, these analyses did not include most recent studies. This analysis aimed to investigate ORR and CR as potential surrogate endpoints for OS.
Methods
A systematic literature review of randomized controlled trials (RCTs) and single-arm studies in RRMM was conducted to identify studies that reported ORR or CR in addition to median OS. Medline, Embase, and Cochrane databases were systematically searched for randomized controlled trials published from 2010 to Jul 2020. Relevant congress abstracts from 2018 to 2020 were also reviewed. Correlations of OS with ORR and with CR were evaluated using Pearson’s product–moment correlation weighted by study sample size. Unadjusted and adjusted weighted linear regression models were constructed to estimate the gain in median OS associated with each surrogate endpoint. Optimal adjusted models considered age, sex, study year, MM type, International Staging System risk stage, prior stem cell transplant, prior lines of therapy, and treatment type.
Results
Sixty-five studies were identified, including 55 treatment arms from 33 RCTs. Moderate-to-strong significant correlations were observed for median OS with ORR (Pearson r=0.79) and CR (r=0.57). The unadjusted absolute analysis models estimated an increase of 0.48 (95% CI: 0.39, 0.57) and 1.94 (95% CI: 1.36, 2.51) months of median OS on average for every additional percentage point of ORR and CR, respectively (Figure 1). Following adjustment for age, relapsed vs refractory MM, and study year, the association was essentially unchanged at 0.46 (95% CI: 0.34, 0.58) and attenuated to 1.17 (95% CI: 0.65, 1.69) months of median OS for each additional point of ORR and CR, respectively.
Conclusion
We demonstrated significant associations of median OS with ORR and CR, supporting the use of ORR and CR as surrogate endpoints for OS benefit in RRMM. Because MM is considered incurable, increasing survival is a key endpoint from both a clinical standpoint and a humanistic standpoint. Being able to predict survival outcomes through endpoints available earlier in a trial may help expedite the approval of novel therapeutics to address this unmet need. In addition, based on unadjusted analysis, there is a clear trend towards higher ORR and CR rates and longer survival among patients with relapsed disease compared with patients with refractory disease. Adjusted models estimated that a 10% increase in ORR predicts a 4.6 month increase in median OS, and a 5% increase in CR predicts a 5.85 month increase in median OS. Often, early phase trials provide initial evidence of treatment benefit, but the follow-up is not long enough to calculate OS. These results suggest that ORR results in initial trials can be used to project survival outcomes, thus potentially informing the design of subsequent trials and accelerating patient care and treatment.
Keyword(s): Multiple myeloma, Survival