
Contributions
Abstract: EP1028
Type: E-Poster Presentation
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
Melphalan flufenamide (melflufen) is an investigational first-in-class peptide-drug conjugate (PDC) that leverages aminopeptidases and thereby rapidly delivers and releases alkylating agents inside tumor cells. It has shown promising clinical activity in relapsed/refractory multiple myeloma (RRMM). In cells, melflufen is rapidly metabolised to produce additional alkylating agents, including melphalan. Since melphalan, in contrast to melflufen, is partially cleared by renal elimination, it is important to assess the impact of renal impairment on melphalan pharmacokinetics (PK), particularly because impaired renal function is a common complication of RRMM.
Aims
The BRIDGE study aims to assess the relationship between renal function and the PK of melphalan in patients (pts) receiving melflufen for RRMM (NCT03639610).
Methods
BRIDGE is a phase 2, multicentre, open-label, single-arm study of pts with RRMM (following 2–4 lines of prior therapy). Informed written consent was obtained from pts with an estimated glomerular filtration rate (eGFR) of ≥30 to <45 ml/min/1.73m2, who received 30 or 40 mg melflufen intravenously on Day 1, and 40 mg oral dexamethasone (20 mg for pts ≥75 yrs) on Days 1, 8, 15, and 22 of each 28-day cycle. PK sampling was performed 3 times in the first 2 cycles: 5–10 mins, 2–3 hrs, and 5–7 hrs after each melflufen infusion. Primary outcome measures were maximum observed concentration (Cmax), area under the concentration versus time curve from 0 hrs to infinity (AUCinf), and elimination phase half-life (t½) of melphalan assessed using non-compartmental methods, and safety. Efficacy and renal function over time were also assessed.
Results
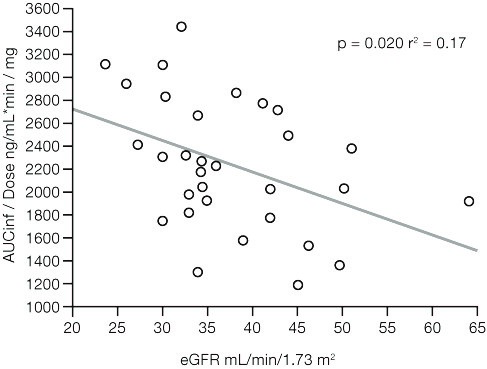
At data cut off (Nov 17, 2020), 31 pts had received melflufen (median age 71 yrs [range 52–85]; 52% male; median eGFR 35 mL/min/1.73m2). Eighteen pts were evaluable for PK analysis. Dose-adjusted AUCinf negatively correlated with eGFR (p = 0.020, r2 = 0.17; Figure) and body surface area (BSA; p = 0.006, r2 = 0.23; combined r2 = 0.44). Dose-adjusted Cmax negatively correlated with BSA (p = 0.0009, r2 = 0.32) and t½ negatively correlated with eGFR. 77% of pts had ≥1 Grade 3/4 treatment-emergent adverse event (TEAE), most commonly thrombocytopenia (58%), neutropenia (42%), and anemia (35%). Seven (23%) pts had ≥1 melflufen-related serious TEAE and 7 (23%) pts had TEAEs leading to melflufen discontinuation. Overall response rate was 48% and clinical benefit rate was 58%. Renal function remained stable; median pre-dose eGFR at Cycle 2 was 42 mL/min/1.73m2.
Conclusion
For pts receiving melflufen in BRIDGE, exposure to melphalan increased with reduced eGFR, similar to what is observed during treatment with melphalan. The overall toxicity profile was as expected for melflufen and dexamethasone and no new safety signals were observed, supporting the use of melflufen in pts with moderate renal impairment. The optimal melflufen dose for pts with impaired renal function will be determined following recruitment of a second cohort of pts with severe renal impairment.
Keyword(s): Melphalan, Multiple myeloma, Pharmacokinetic, Renal impairment
Abstract: EP1028
Type: E-Poster Presentation
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
Melphalan flufenamide (melflufen) is an investigational first-in-class peptide-drug conjugate (PDC) that leverages aminopeptidases and thereby rapidly delivers and releases alkylating agents inside tumor cells. It has shown promising clinical activity in relapsed/refractory multiple myeloma (RRMM). In cells, melflufen is rapidly metabolised to produce additional alkylating agents, including melphalan. Since melphalan, in contrast to melflufen, is partially cleared by renal elimination, it is important to assess the impact of renal impairment on melphalan pharmacokinetics (PK), particularly because impaired renal function is a common complication of RRMM.
Aims
The BRIDGE study aims to assess the relationship between renal function and the PK of melphalan in patients (pts) receiving melflufen for RRMM (NCT03639610).
Methods
BRIDGE is a phase 2, multicentre, open-label, single-arm study of pts with RRMM (following 2–4 lines of prior therapy). Informed written consent was obtained from pts with an estimated glomerular filtration rate (eGFR) of ≥30 to <45 ml/min/1.73m2, who received 30 or 40 mg melflufen intravenously on Day 1, and 40 mg oral dexamethasone (20 mg for pts ≥75 yrs) on Days 1, 8, 15, and 22 of each 28-day cycle. PK sampling was performed 3 times in the first 2 cycles: 5–10 mins, 2–3 hrs, and 5–7 hrs after each melflufen infusion. Primary outcome measures were maximum observed concentration (Cmax), area under the concentration versus time curve from 0 hrs to infinity (AUCinf), and elimination phase half-life (t½) of melphalan assessed using non-compartmental methods, and safety. Efficacy and renal function over time were also assessed.
Results
At data cut off (Nov 17, 2020), 31 pts had received melflufen (median age 71 yrs [range 52–85]; 52% male; median eGFR 35 mL/min/1.73m2). Eighteen pts were evaluable for PK analysis. Dose-adjusted AUCinf negatively correlated with eGFR (p = 0.020, r2 = 0.17; Figure) and body surface area (BSA; p = 0.006, r2 = 0.23; combined r2 = 0.44). Dose-adjusted Cmax negatively correlated with BSA (p = 0.0009, r2 = 0.32) and t½ negatively correlated with eGFR. 77% of pts had ≥1 Grade 3/4 treatment-emergent adverse event (TEAE), most commonly thrombocytopenia (58%), neutropenia (42%), and anemia (35%). Seven (23%) pts had ≥1 melflufen-related serious TEAE and 7 (23%) pts had TEAEs leading to melflufen discontinuation. Overall response rate was 48% and clinical benefit rate was 58%. Renal function remained stable; median pre-dose eGFR at Cycle 2 was 42 mL/min/1.73m2.

Conclusion
For pts receiving melflufen in BRIDGE, exposure to melphalan increased with reduced eGFR, similar to what is observed during treatment with melphalan. The overall toxicity profile was as expected for melflufen and dexamethasone and no new safety signals were observed, supporting the use of melflufen in pts with moderate renal impairment. The optimal melflufen dose for pts with impaired renal function will be determined following recruitment of a second cohort of pts with severe renal impairment.
Keyword(s): Melphalan, Multiple myeloma, Pharmacokinetic, Renal impairment


