
Contributions
Abstract: EP1027
Type: E-Poster Presentation
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
Frailty in non-transplant eligible (NTE) newly diagnosed multiple myeloma (NDMM) patients is associated with non-hematological toxicity which can negatively affect physical functioning (PF). Older patients increasingly prefer quality of life (QoL) and physical independence over length of life, highlighting the importance of health-related (HR)QoL assessment to take into account for treatment guidance. We here present the HRQoL results of the HOVON 123 trial. In addition to previous reports on HRQOL with MPV, we here present results by frailty level and also after completion of treatment for the first time.
Aims
To evaluate 2 year HRQoL in unfit and frail patients treated with MPV in the HOVON123 trial.
Methods
In this prospective phase 2 trial patients ≥75 years were treated with 9 dose-adjusted cycles MPV. Two HRQoL instruments (EORTC QLQ-C30 and MY20) were obtained at baseline (T0), after 3 (T1) and 9 (T2) cycles and after 6 (T3) and 12 (T4) months of follow-up for patients on protocol. Nine (3 functional; 6 symptom) subscales were presented as concepts relevant to patients: global QoL (GHS), PF, future perspective; fatigue, pain, constipation, diarrhoea, treatment side effects, and neuropathy (PNP, based on “tingling hands/feet” Q13 MY20). Baseline differences were analysed with independent t-tests, changes over time with linear mixed models. Minimal important difference (MID) from T0 within groups was defined as a score change of ≥1 SEM for multi-item scales or ≥0.5xSD for single-item scales. Cross-sectional clinically relevant superiority of one group was defined as MID≥5 between groups. HRQOL changes/differences were reported only when both statistically significant and clinically relevant (>MID). Analyses were based on only unfit and frail patients (based on IMWG-frailty index (Palumbo et al, 2015)).
Results
Of 238 included patients, 71 (30%) were unfit and 130 (55%) frail. Of those, 67 (94%) and 130 (100%) completed a baseline questionnaire. Compliance during T1-T4 was comparable between unfit and frail, ranging from 59-92%.
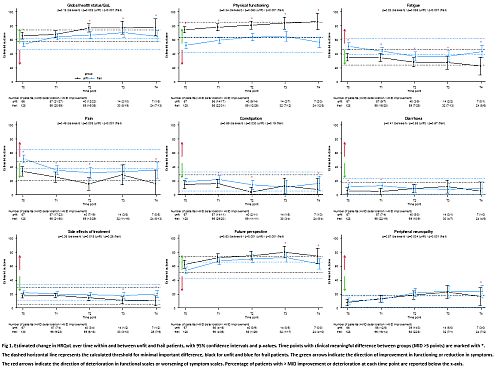
At baseline, HRQoL was generally superior in unfit vs frail patients, which remained over time (Fig 1). In contrast, there was no difference in constipation, side effects and PNP between unfit and frail (Fig 1). When looking at unfit and frail patients separately, we observed that GHS, future perspective and pain improved, whereas PNP deteriorated in both groups. In addition, in frail patients also PF and fatigue improved, and in unfit patients constipation improved. Improvement in GHS, future perspective, pain, PF and fatigue was reached earlier in frail as compared to unfit patients (Fig 1). Importantly, these improvements were sustained after treatment completion. However, in frail patients a decrease in PF, fatigue and future perspective as compared to earlier time points was observed at T4 but still superior compared to baseline. With respect to PNP, a clinically relevant worsening was observed in both unfit and frail patients from T2 onwards.
Conclusion
In this first study reporting HRQoL in NDMM patients by frailty level, we show that unfit had a superior HRQoL as compared to frail, both at baseline and follow-up. A HRQoL improvement over time was not only observed in unfit (4/9 scales) but importantly, also in the frail (5/9 scales) patients, who even showed an improvement at earlier time points. Only PNP worsened over time in both groups, as expected because of bortezomib treatment, however not negatively affecting physical functioning in those patients who completed 9 MPV-cycles.
Keyword(s): Elderly, Multiple myeloma, Quality of life
Abstract: EP1027
Type: E-Poster Presentation
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
Frailty in non-transplant eligible (NTE) newly diagnosed multiple myeloma (NDMM) patients is associated with non-hematological toxicity which can negatively affect physical functioning (PF). Older patients increasingly prefer quality of life (QoL) and physical independence over length of life, highlighting the importance of health-related (HR)QoL assessment to take into account for treatment guidance. We here present the HRQoL results of the HOVON 123 trial. In addition to previous reports on HRQOL with MPV, we here present results by frailty level and also after completion of treatment for the first time.
Aims
To evaluate 2 year HRQoL in unfit and frail patients treated with MPV in the HOVON123 trial.
Methods
In this prospective phase 2 trial patients ≥75 years were treated with 9 dose-adjusted cycles MPV. Two HRQoL instruments (EORTC QLQ-C30 and MY20) were obtained at baseline (T0), after 3 (T1) and 9 (T2) cycles and after 6 (T3) and 12 (T4) months of follow-up for patients on protocol. Nine (3 functional; 6 symptom) subscales were presented as concepts relevant to patients: global QoL (GHS), PF, future perspective; fatigue, pain, constipation, diarrhoea, treatment side effects, and neuropathy (PNP, based on “tingling hands/feet” Q13 MY20). Baseline differences were analysed with independent t-tests, changes over time with linear mixed models. Minimal important difference (MID) from T0 within groups was defined as a score change of ≥1 SEM for multi-item scales or ≥0.5xSD for single-item scales. Cross-sectional clinically relevant superiority of one group was defined as MID≥5 between groups. HRQOL changes/differences were reported only when both statistically significant and clinically relevant (>MID). Analyses were based on only unfit and frail patients (based on IMWG-frailty index (Palumbo et al, 2015)).
Results
Of 238 included patients, 71 (30%) were unfit and 130 (55%) frail. Of those, 67 (94%) and 130 (100%) completed a baseline questionnaire. Compliance during T1-T4 was comparable between unfit and frail, ranging from 59-92%.
At baseline, HRQoL was generally superior in unfit vs frail patients, which remained over time (Fig 1). In contrast, there was no difference in constipation, side effects and PNP between unfit and frail (Fig 1). When looking at unfit and frail patients separately, we observed that GHS, future perspective and pain improved, whereas PNP deteriorated in both groups. In addition, in frail patients also PF and fatigue improved, and in unfit patients constipation improved. Improvement in GHS, future perspective, pain, PF and fatigue was reached earlier in frail as compared to unfit patients (Fig 1). Importantly, these improvements were sustained after treatment completion. However, in frail patients a decrease in PF, fatigue and future perspective as compared to earlier time points was observed at T4 but still superior compared to baseline. With respect to PNP, a clinically relevant worsening was observed in both unfit and frail patients from T2 onwards.

Conclusion
In this first study reporting HRQoL in NDMM patients by frailty level, we show that unfit had a superior HRQoL as compared to frail, both at baseline and follow-up. A HRQoL improvement over time was not only observed in unfit (4/9 scales) but importantly, also in the frail (5/9 scales) patients, who even showed an improvement at earlier time points. Only PNP worsened over time in both groups, as expected because of bortezomib treatment, however not negatively affecting physical functioning in those patients who completed 9 MPV-cycles.
Keyword(s): Elderly, Multiple myeloma, Quality of life


