
Contributions
Abstract: EP1019
Type: E-Poster Presentation
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
Lenalidomide (LEN) with or without a proteasome inhibitor (PI) administered until disease progression is a standard treatment (Tx) approach for patients with newly diagnosed multiple myeloma (MM). However, recent clinical trials investigating triplet therapies did not have a large representation of patients whose disease has relapsed or become refractory to LEN after early-line Tx. The phase 2 MM-014 trial (NCT01946477) was designed to investigate the outcomes with sequencing pomalidomide (POM)-based therapy immediately after first- or second-line LEN-based Tx failure in patients with relapsed refractory MM (RRMM). In an earlier report of the intention-to-treat population (N = 112) from cohort B of this trial, POM + dexamethasone (DEX) + daratumumab (DARA) demonstrated promising efficacy and safety with an overall response rate (ORR) of 77.7%, and median progression-free survival (PFS) of 30.8 months (Siegel DS, et al. Blood 2020;136[suppl 1]: 16–17).
Aims
To report the efficacy and safety of POM + DEX + DARA immediately after LEN-based Tx failure in a subgroup of cohort B patients who received prior LEN + PI.
Methods
Patients with RRMM treated with 1-2 prior Tx lines, LEN-based Tx for ≥ 2 consecutive cycles as their most recent regimen, and progressive disease during or after their last line of Tx received POM + DEX + DARA. POM 4 mg/d was given orally on days 1-21; DEX 40 mg/d (20 mg/d in patients aged > 75 years) was given orally on days 1, 8, 15, and 22; and DARA 16 mg/kg was given intravenously on days 1, 8, 15, and 22 of cycles 1 and 2, days 1 and 15 for cycles 3-6, and day 1 for cycles 7+. The primary endpoint was ORR; secondary endpoints included PFS and safety.
Results
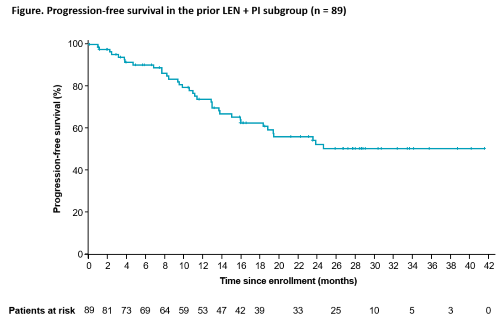
In cohort B, 89 of 112 patients (79%; median age, 65.0 years) had received prior LEN + PI. Among this patient subgroup, 67 patients (75.3%) had LEN-refractory MM and 22 (24.7%) had MM that relapsed after LEN Tx; most patients (52 [58.4%]) received 1 vs 2 (37 [41.6%]) prior Tx lines. Median duration of treatment of POM, DEX, and DARA was 15.9. 14.0, and 16.1 months, respectively. At data cutoff (March 24, 2020), the ORR was 78.7% (≥ very good partial response [VGPR], 55.1%); median follow-up of 28.3 months. The ORR was 80.8% (≥ VGPR, 63.5%) and 75.7% (≥ VGPR, 43.2%) in patients with 1 vs 2 prior lines of treatment, respectively. Median duration of response and median PFS were not yet reached (Figure; 1-year PFS rate, 74.1%). Infusion-related reactions occurred in 28.1% of patients and were primarily low grade. Overall, 97.8% of patients had ≥ 1 grade 3/4 Tx-emergent adverse event. The most common grade 3/4 hematologic events were neutropenia (64.0%; febrile, 12.4%), anemia (19.1%), and thrombocytopenia (13.5%). Grade 3/4 infections occurred in 40.4% of patients, including 15.7% with grade 3/4 pneumonia.
Conclusion
In this subanalysis, POM + DEX + DARA administered immediately after LEN failure in a subgroup of patients previously treated with LEN + PI demonstrated a high response rate and a safety profile consistent with that in the overall cohort B population. The results support the use of POM-based therapy, integrating the monoclonal antibody DARA as early as second line in patients with RRMM, potentially immediately after LEN Tx failure. Furthermore, these data support subsequent Tx with the same immunomodulatory agent class in a patient population with LEN-relapsed or -refractory disease.
Keyword(s): Clinical trial, Multiple myeloma, Refractory, Relapse
Abstract: EP1019
Type: E-Poster Presentation
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
Lenalidomide (LEN) with or without a proteasome inhibitor (PI) administered until disease progression is a standard treatment (Tx) approach for patients with newly diagnosed multiple myeloma (MM). However, recent clinical trials investigating triplet therapies did not have a large representation of patients whose disease has relapsed or become refractory to LEN after early-line Tx. The phase 2 MM-014 trial (NCT01946477) was designed to investigate the outcomes with sequencing pomalidomide (POM)-based therapy immediately after first- or second-line LEN-based Tx failure in patients with relapsed refractory MM (RRMM). In an earlier report of the intention-to-treat population (N = 112) from cohort B of this trial, POM + dexamethasone (DEX) + daratumumab (DARA) demonstrated promising efficacy and safety with an overall response rate (ORR) of 77.7%, and median progression-free survival (PFS) of 30.8 months (Siegel DS, et al. Blood 2020;136[suppl 1]: 16–17).
Aims
To report the efficacy and safety of POM + DEX + DARA immediately after LEN-based Tx failure in a subgroup of cohort B patients who received prior LEN + PI.
Methods
Patients with RRMM treated with 1-2 prior Tx lines, LEN-based Tx for ≥ 2 consecutive cycles as their most recent regimen, and progressive disease during or after their last line of Tx received POM + DEX + DARA. POM 4 mg/d was given orally on days 1-21; DEX 40 mg/d (20 mg/d in patients aged > 75 years) was given orally on days 1, 8, 15, and 22; and DARA 16 mg/kg was given intravenously on days 1, 8, 15, and 22 of cycles 1 and 2, days 1 and 15 for cycles 3-6, and day 1 for cycles 7+. The primary endpoint was ORR; secondary endpoints included PFS and safety.
Results
In cohort B, 89 of 112 patients (79%; median age, 65.0 years) had received prior LEN + PI. Among this patient subgroup, 67 patients (75.3%) had LEN-refractory MM and 22 (24.7%) had MM that relapsed after LEN Tx; most patients (52 [58.4%]) received 1 vs 2 (37 [41.6%]) prior Tx lines. Median duration of treatment of POM, DEX, and DARA was 15.9. 14.0, and 16.1 months, respectively. At data cutoff (March 24, 2020), the ORR was 78.7% (≥ very good partial response [VGPR], 55.1%); median follow-up of 28.3 months. The ORR was 80.8% (≥ VGPR, 63.5%) and 75.7% (≥ VGPR, 43.2%) in patients with 1 vs 2 prior lines of treatment, respectively. Median duration of response and median PFS were not yet reached (Figure; 1-year PFS rate, 74.1%). Infusion-related reactions occurred in 28.1% of patients and were primarily low grade. Overall, 97.8% of patients had ≥ 1 grade 3/4 Tx-emergent adverse event. The most common grade 3/4 hematologic events were neutropenia (64.0%; febrile, 12.4%), anemia (19.1%), and thrombocytopenia (13.5%). Grade 3/4 infections occurred in 40.4% of patients, including 15.7% with grade 3/4 pneumonia.

Conclusion
In this subanalysis, POM + DEX + DARA administered immediately after LEN failure in a subgroup of patients previously treated with LEN + PI demonstrated a high response rate and a safety profile consistent with that in the overall cohort B population. The results support the use of POM-based therapy, integrating the monoclonal antibody DARA as early as second line in patients with RRMM, potentially immediately after LEN Tx failure. Furthermore, these data support subsequent Tx with the same immunomodulatory agent class in a patient population with LEN-relapsed or -refractory disease.
Keyword(s): Clinical trial, Multiple myeloma, Refractory, Relapse


