
Contributions
Abstract: EP1016
Type: E-Poster Presentation
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
Severe renal impairment (RI) is a common myeloma (MM) complication. For patients with relapsed/refractory MM (RRMM) there is a need for effective therapies with low or minimal risk of renal toxicity. Daratumumab (DARA), an IgG1κ human monoclonal antibody that targets CD38, has shown durable responses and a favorable safety profile in heavily pretreated patients (pts) with RRMM. However, there is limited data on the safety and efficacy of DARA in pts with RRMM and severe RI.
Aims
DARE study aims to assess the safety and efficacy of DARA with dexamethasone in pts with RRMM and severe RI.
Methods
DARE is a prospective, open-label, multicenter, phase 2 study conducted in 7 sites in Greece and Italy, and has completed enrolment. Eligible adult pts had documented RRMM and severe RI (estimated glomerular filtration rate [eGFR] <30 mL/min/1.73m2 or requiring dialysis), who had ≥2 previous treatments of both bortezomib- and lenalidomide-based regimens, had progressive disease after their last treatment and Eastern Cooperative Oncology Group performance status (ECOG PS) ≤2. Exclusion criteria included previous daratumumab or other anti-CD38 therapy. Pts received 28-day treatment cycles with 16 mg/kg intravenous daratumumab (weekly for cycles 1–2, every 2 weeks for cycles 3–6, and every 4 weeks thereafter) and oral dexamethasone (40 mg weekly). The primary endpoint is progression-free survival (PFS). Secondary endpoints include overall response rate (ORR; partial response [PR] or better), renal response rate (RRR; best response of renal partial response or better), and safety. All responses were based on investigators’ assessment as per IMWG criteria.
Results
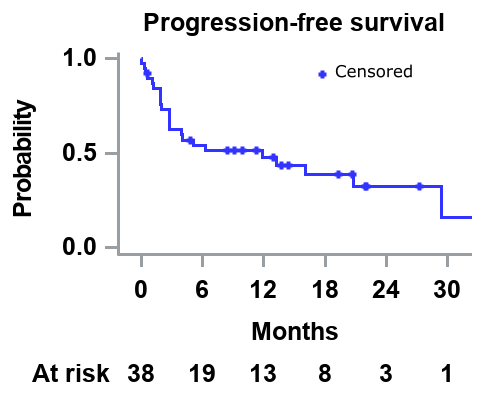
In total, 38 pts (male: 76.3%; median age: 72 years; median time from diagnosis to study entry: 4.2 years) were included. The median number of prior therapies was 3, and 34.2% had undergone autologous stem cell transplantation. At study entry, 10.5% and 89.5% of pts had International Staging System (ISS) 2 and 3 disease, median eGFR was 13 mL/min/1.73 m2, and 44.7% were on dialysis. The median follow-up is 10 months, and pts received a median of 8 cycles of DARA with dexamethasone. The 6-month and median PFS is 54% and 11.8 months respectively (Figure). The ORR was 47.4% (very good partial response: 34.2%, PR: 13.2%), and the ORR for pts on dialysis (n=17) was 47.1%. The median time from the first study treatment dose to first response (PR or better) was 0.9 months. The RRR was 15.8%. By the cut-off date (31/12/2020) 31.6% of pts were still receiving protocol therapy; 68.4% had discontinued treatment (progressive disease: 36.8%; lost to follow-up: 7.9%; patient choice 5.2%, and SAE: 18.4%). Overall, 50% of pts had ≥1 adverse event (AE) grade 3/4 (most common were anemia [15.8%] and hyperglycemia [13.2%]), and 26.3% (n=10) had ≥1 serious adverse event (SAE), mostly infectious complications: septic shock (n=3, all fatal), pneumonia, peritonitis (fatal), lower respiratory tract infection (fatal), ECOG PS worsened (fatal), cerebrovascular accident, myocardial infarction (fatal), acute kidney injury, and hyperkalemia (n=1 each).
Conclusion
DARA and dexamethasone is an effective treatment for RRMM with severe renal dysfunction or requiring dialysis, associated with rapid and high rates of hematologic and respectable rates of renal responses in this heavily pretreated population. Notably, responses were similar in pts regardless of being on dialysis or not. No new safety signals were observed but antibiotic prophylaxis may potentially reduce infection risk.
Keyword(s): Multiple myeloma, Renal impairment
Abstract: EP1016
Type: E-Poster Presentation
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
Severe renal impairment (RI) is a common myeloma (MM) complication. For patients with relapsed/refractory MM (RRMM) there is a need for effective therapies with low or minimal risk of renal toxicity. Daratumumab (DARA), an IgG1κ human monoclonal antibody that targets CD38, has shown durable responses and a favorable safety profile in heavily pretreated patients (pts) with RRMM. However, there is limited data on the safety and efficacy of DARA in pts with RRMM and severe RI.
Aims
DARE study aims to assess the safety and efficacy of DARA with dexamethasone in pts with RRMM and severe RI.
Methods
DARE is a prospective, open-label, multicenter, phase 2 study conducted in 7 sites in Greece and Italy, and has completed enrolment. Eligible adult pts had documented RRMM and severe RI (estimated glomerular filtration rate [eGFR] <30 mL/min/1.73m2 or requiring dialysis), who had ≥2 previous treatments of both bortezomib- and lenalidomide-based regimens, had progressive disease after their last treatment and Eastern Cooperative Oncology Group performance status (ECOG PS) ≤2. Exclusion criteria included previous daratumumab or other anti-CD38 therapy. Pts received 28-day treatment cycles with 16 mg/kg intravenous daratumumab (weekly for cycles 1–2, every 2 weeks for cycles 3–6, and every 4 weeks thereafter) and oral dexamethasone (40 mg weekly). The primary endpoint is progression-free survival (PFS). Secondary endpoints include overall response rate (ORR; partial response [PR] or better), renal response rate (RRR; best response of renal partial response or better), and safety. All responses were based on investigators’ assessment as per IMWG criteria.
Results
In total, 38 pts (male: 76.3%; median age: 72 years; median time from diagnosis to study entry: 4.2 years) were included. The median number of prior therapies was 3, and 34.2% had undergone autologous stem cell transplantation. At study entry, 10.5% and 89.5% of pts had International Staging System (ISS) 2 and 3 disease, median eGFR was 13 mL/min/1.73 m2, and 44.7% were on dialysis. The median follow-up is 10 months, and pts received a median of 8 cycles of DARA with dexamethasone. The 6-month and median PFS is 54% and 11.8 months respectively (Figure). The ORR was 47.4% (very good partial response: 34.2%, PR: 13.2%), and the ORR for pts on dialysis (n=17) was 47.1%. The median time from the first study treatment dose to first response (PR or better) was 0.9 months. The RRR was 15.8%. By the cut-off date (31/12/2020) 31.6% of pts were still receiving protocol therapy; 68.4% had discontinued treatment (progressive disease: 36.8%; lost to follow-up: 7.9%; patient choice 5.2%, and SAE: 18.4%). Overall, 50% of pts had ≥1 adverse event (AE) grade 3/4 (most common were anemia [15.8%] and hyperglycemia [13.2%]), and 26.3% (n=10) had ≥1 serious adverse event (SAE), mostly infectious complications: septic shock (n=3, all fatal), pneumonia, peritonitis (fatal), lower respiratory tract infection (fatal), ECOG PS worsened (fatal), cerebrovascular accident, myocardial infarction (fatal), acute kidney injury, and hyperkalemia (n=1 each).

Conclusion
DARA and dexamethasone is an effective treatment for RRMM with severe renal dysfunction or requiring dialysis, associated with rapid and high rates of hematologic and respectable rates of renal responses in this heavily pretreated population. Notably, responses were similar in pts regardless of being on dialysis or not. No new safety signals were observed but antibiotic prophylaxis may potentially reduce infection risk.
Keyword(s): Multiple myeloma, Renal impairment


