
Contributions
Abstract: EP1014
Type: E-Poster Presentation
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
Second-line treatment of patients (pts) with multiple myeloma (MM), who are refractory to, or have relapsed after frontline lenalidomide-based therapy is challenging. Daratumumab (DARA), an anti-CD38 monoclonal antibody, has shown significant clinical activity and an acceptable safety profile in pts with relapsed/refractory multiple myeloma (RRMM) as monotherapy or in combination with other agents. Ixazomib, the first oral proteasome inhibitor, has also been shown to be efficacious and with a positive safety profile in newly diagnosed MM pts.
Aims
This study aims to evaluate the effectiveness of DARA in combination with ixazomib and dexamethasone (DId) as second-line therapy in pts with RRMM who have previously been treated with a lenalidomide-based regimen.
Methods
DARIA is an ongoing, prospective, open-label, multicenter, phase 2 study. Eligible adult pts with RRMM had measurable disease after 1 prior line with a lenalidomide-based regimen and a Karnofsky Performance Status (KPS) score of ≥70. Exclusion criteria included previous DARA or anti-CD38 or ixazomib treatment. Treatment with DId comprises an induction phase of 9 cycles (each of 28 days in duration) and a maintenance phase. In induction, pts received DARA 16mg/kg (weekly for cycles 1–2, every 2 weeks for cycles 3–6, and every 4 weeks thereafter) administered intravenously until November 2020 and subcutaneously at a fixed dose of 1800 mg thereafter; 4mg oral ixazomib (days 1, 8, and 15 of each cycle); and 40mg oral dexamethasone (weekly, each cycle). In maintenance, DARA and ixazomib are administered every 4 weeks until disease progression or unacceptable toxicity, with dexamethasone being discontinued. The primary endpoint is overall response rate (ORR). This report presents results for pts who received the first dose at least 6 months prior to the cut-off date (31/12/2020).
Results
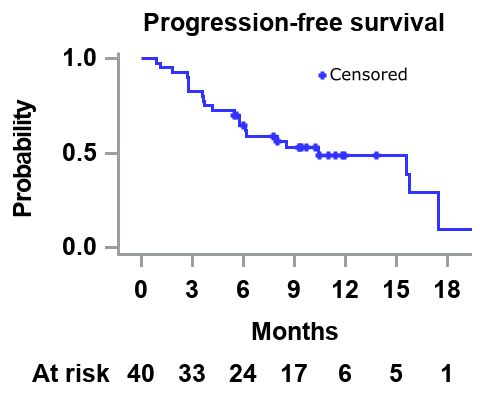
This analysis includes 40 pts (median age: 69.0 years; male: 52.5%). At screening, 75.0% of pts had a KPS score of ≥90. 50.0%, 32.5%, and 17.5% had an International Staging System (ISS) stage of 1, 2, and 3, respectively; and 37.5%, 52.5%, and 7.5% had a revised ISS of 1, 2, and 3, respectively. 70.0% of pts were refractory to lenalidomide, and 37.5% of them had received prior autologous stem cell transplantation. The median time from diagnosis to first study dose was 2.1 years. Up to the cut-off date, pts received a median of 9 treatment cycles. ORR was 57.5% (complete response: 2.5%, VGPR: 30.0%, and PR: 25.0%). The median time from first DId dose until first response (PR or better) was 0.9 months. The median PFS was 10.4 months (figure), and 37.5% of the pts were still on treatment by the cut-off date. Of all pts, 62.5% discontinued treatment (40% due to progressive disease, 12.5% due to physician’s decision, and 10% due to a safety event). Overall, 40% of the pts had ≥1 AE grade 3/4, the most common being thrombocytopenia (n=8, 20.0%), and 25.0% (n=10) had ≥1 serious adverse event, the most common being acute kidney injury (n=2, 5.0%) and pneumonia (n=2, 5.0%, one of which fatal). In total, there were 3 fatal SAE, the other 2 being infection and a lower respiratory tract infection.
Conclusion
Second-line treatment with DId in pts with RRMM who were previously treated with a lenalidomide-based regimen resulted in rapid (median time to PR or better was less than a month) and deep responses (about a third of pts exhibited VGPR or better). The safety profile of DId was very good, and the majority of pts (60%) did not experience grade 3/4 AE, whereas 25% of them experienced a SAE.
Keyword(s): Multiple myeloma
Abstract: EP1014
Type: E-Poster Presentation
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
Second-line treatment of patients (pts) with multiple myeloma (MM), who are refractory to, or have relapsed after frontline lenalidomide-based therapy is challenging. Daratumumab (DARA), an anti-CD38 monoclonal antibody, has shown significant clinical activity and an acceptable safety profile in pts with relapsed/refractory multiple myeloma (RRMM) as monotherapy or in combination with other agents. Ixazomib, the first oral proteasome inhibitor, has also been shown to be efficacious and with a positive safety profile in newly diagnosed MM pts.
Aims
This study aims to evaluate the effectiveness of DARA in combination with ixazomib and dexamethasone (DId) as second-line therapy in pts with RRMM who have previously been treated with a lenalidomide-based regimen.
Methods
DARIA is an ongoing, prospective, open-label, multicenter, phase 2 study. Eligible adult pts with RRMM had measurable disease after 1 prior line with a lenalidomide-based regimen and a Karnofsky Performance Status (KPS) score of ≥70. Exclusion criteria included previous DARA or anti-CD38 or ixazomib treatment. Treatment with DId comprises an induction phase of 9 cycles (each of 28 days in duration) and a maintenance phase. In induction, pts received DARA 16mg/kg (weekly for cycles 1–2, every 2 weeks for cycles 3–6, and every 4 weeks thereafter) administered intravenously until November 2020 and subcutaneously at a fixed dose of 1800 mg thereafter; 4mg oral ixazomib (days 1, 8, and 15 of each cycle); and 40mg oral dexamethasone (weekly, each cycle). In maintenance, DARA and ixazomib are administered every 4 weeks until disease progression or unacceptable toxicity, with dexamethasone being discontinued. The primary endpoint is overall response rate (ORR). This report presents results for pts who received the first dose at least 6 months prior to the cut-off date (31/12/2020).
Results
This analysis includes 40 pts (median age: 69.0 years; male: 52.5%). At screening, 75.0% of pts had a KPS score of ≥90. 50.0%, 32.5%, and 17.5% had an International Staging System (ISS) stage of 1, 2, and 3, respectively; and 37.5%, 52.5%, and 7.5% had a revised ISS of 1, 2, and 3, respectively. 70.0% of pts were refractory to lenalidomide, and 37.5% of them had received prior autologous stem cell transplantation. The median time from diagnosis to first study dose was 2.1 years. Up to the cut-off date, pts received a median of 9 treatment cycles. ORR was 57.5% (complete response: 2.5%, VGPR: 30.0%, and PR: 25.0%). The median time from first DId dose until first response (PR or better) was 0.9 months. The median PFS was 10.4 months (figure), and 37.5% of the pts were still on treatment by the cut-off date. Of all pts, 62.5% discontinued treatment (40% due to progressive disease, 12.5% due to physician’s decision, and 10% due to a safety event). Overall, 40% of the pts had ≥1 AE grade 3/4, the most common being thrombocytopenia (n=8, 20.0%), and 25.0% (n=10) had ≥1 serious adverse event, the most common being acute kidney injury (n=2, 5.0%) and pneumonia (n=2, 5.0%, one of which fatal). In total, there were 3 fatal SAE, the other 2 being infection and a lower respiratory tract infection.

Conclusion
Second-line treatment with DId in pts with RRMM who were previously treated with a lenalidomide-based regimen resulted in rapid (median time to PR or better was less than a month) and deep responses (about a third of pts exhibited VGPR or better). The safety profile of DId was very good, and the majority of pts (60%) did not experience grade 3/4 AE, whereas 25% of them experienced a SAE.
Keyword(s): Multiple myeloma


