
Contributions
Abstract: EP1013
Type: E-Poster Presentation
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
Pomalidomide (POM)-based combination(s) are a standard therapy for patients with lenalidomide (LEN)-exposed relapsed or refractory multiple myeloma (RRMM). Multiple studies have assessed the efficacy and risk profile of POM-containing regimens, leading to the approval of regimens such as PVd, DPd, and EloPd.
Aims
To conduct a meta-analysis of progression-free survival (PFS) outcomes reported for POM-based combination regimens and generate a pooled efficacy estimate.
Methods
A targeted search was conducted to identify studies between 2016 and 2020 in patients previously treated with LEN. Trials had to include a POM-based combination with proteasome inhibitors (PVd, KPd), monoclonal antibodies (EloPd, DPd, IsaPd) or an alkylating agent (PCd). Kaplan-Meier curves reported in publications were digitized to reconstruct the individual patient data (IPD) through the Guyot algorithm. Reconstructed IPD were then used to generate survival analyses and obtain a detailed description of individual survival curves. Individual curves were pooled using a random-effects model and the DerSimonian and Laird method. The I2 statistic was used to test for heterogeneity between the studies, with 0% suggesting the lowest level of heterogeneity. Heterogeneity was observed between studies in terms of patient characteristics and number of prior lines of therapies. Three unadjusted analyses were performed: a meta-analysis to obtain a pooled median PFS based on all the studies, independent of the number of prior lines; a meta-analysis restricted to studies that included 2L patients (2L+); and a meta-analysis of PFS reported in 2L patients only. The 1- and 2-year PFS rates were then extracted.
Results
Five randomized controlled trials (RCTs) (OPTIMISMM—PVd vs Vd, APOLLO—DPd vs Pd, ELOQUENT-3—EloPd vs Pd, ICARIA-MM—IsaPd vs Pd, and NCT01432600—Cd vs Pd) and 6 single-arm trials (MM-014—DPd, MMRC—KPd, NCT02185820—KPd, NCT02176213—PCd, EMN1—KPd, and IFM2013—PCd) were identified. Eleven, 7, and 3 trials were included, with heterogeneity levels estimated at 15.3%, 13.2% and 0.0%, respectively. The overall population was found to have a median PFS of 14.1 months (Table). The unadjusted pooled median PFS in trials including 2L+ patients was 16.9 months. Finally, when considering only 2L patients, the pooled median PFS was 21.3 months. The PFS rates at 1 and 2 years were 57% and 30%, respectively, in the overall population, 62% and 39% for trials including 2L+ patients, and 74% and 42% when considering only 2L patients. Although unadjusted, the pooled estimated median PFS highlighted that POM-based combinations seem to have better efficacy when used in early treatment lines.
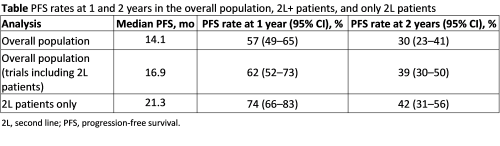
Conclusion
POM-based combinations have been an effective treatment strategy in RRMM post LEN. The efficacy associated with these combinations was found to be higher for patients treated in earlier lines (second line) compared with the overall population of previously treated patients. POM delivered consistent efficacy and synergy in combinations with protease inhibitors and monoclonal antibodies. These data suggest that class switch may not always be necessary when making treatment decisions.
Keyword(s): Clinical outcome, Meta-analysis, Multiple myeloma, Treatment
Abstract: EP1013
Type: E-Poster Presentation
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
Pomalidomide (POM)-based combination(s) are a standard therapy for patients with lenalidomide (LEN)-exposed relapsed or refractory multiple myeloma (RRMM). Multiple studies have assessed the efficacy and risk profile of POM-containing regimens, leading to the approval of regimens such as PVd, DPd, and EloPd.
Aims
To conduct a meta-analysis of progression-free survival (PFS) outcomes reported for POM-based combination regimens and generate a pooled efficacy estimate.
Methods
A targeted search was conducted to identify studies between 2016 and 2020 in patients previously treated with LEN. Trials had to include a POM-based combination with proteasome inhibitors (PVd, KPd), monoclonal antibodies (EloPd, DPd, IsaPd) or an alkylating agent (PCd). Kaplan-Meier curves reported in publications were digitized to reconstruct the individual patient data (IPD) through the Guyot algorithm. Reconstructed IPD were then used to generate survival analyses and obtain a detailed description of individual survival curves. Individual curves were pooled using a random-effects model and the DerSimonian and Laird method. The I2 statistic was used to test for heterogeneity between the studies, with 0% suggesting the lowest level of heterogeneity. Heterogeneity was observed between studies in terms of patient characteristics and number of prior lines of therapies. Three unadjusted analyses were performed: a meta-analysis to obtain a pooled median PFS based on all the studies, independent of the number of prior lines; a meta-analysis restricted to studies that included 2L patients (2L+); and a meta-analysis of PFS reported in 2L patients only. The 1- and 2-year PFS rates were then extracted.
Results
Five randomized controlled trials (RCTs) (OPTIMISMM—PVd vs Vd, APOLLO—DPd vs Pd, ELOQUENT-3—EloPd vs Pd, ICARIA-MM—IsaPd vs Pd, and NCT01432600—Cd vs Pd) and 6 single-arm trials (MM-014—DPd, MMRC—KPd, NCT02185820—KPd, NCT02176213—PCd, EMN1—KPd, and IFM2013—PCd) were identified. Eleven, 7, and 3 trials were included, with heterogeneity levels estimated at 15.3%, 13.2% and 0.0%, respectively. The overall population was found to have a median PFS of 14.1 months (Table). The unadjusted pooled median PFS in trials including 2L+ patients was 16.9 months. Finally, when considering only 2L patients, the pooled median PFS was 21.3 months. The PFS rates at 1 and 2 years were 57% and 30%, respectively, in the overall population, 62% and 39% for trials including 2L+ patients, and 74% and 42% when considering only 2L patients. Although unadjusted, the pooled estimated median PFS highlighted that POM-based combinations seem to have better efficacy when used in early treatment lines.
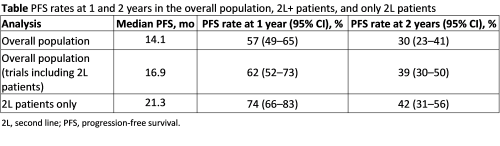
Conclusion
POM-based combinations have been an effective treatment strategy in RRMM post LEN. The efficacy associated with these combinations was found to be higher for patients treated in earlier lines (second line) compared with the overall population of previously treated patients. POM delivered consistent efficacy and synergy in combinations with protease inhibitors and monoclonal antibodies. These data suggest that class switch may not always be necessary when making treatment decisions.
Keyword(s): Clinical outcome, Meta-analysis, Multiple myeloma, Treatment


