
Contributions
Abstract: EP1011
Type: E-Poster Presentation
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
Systemic light chain (AL) amyloidosis is a serious plasma cell disorder where amyloid is deposited in various organs and tissues throughout the body, disrupting their function, and severely affecting the quality of life of the patients (pts). Therapeutic regimens have been used off-label until very recently, therefore leading to differences in treatment patterns across Europe.
Aims
The current analysis aims to describe differences in patient characteristics and treatment strategies among different European countries in the recent years.
Methods
EMN23 is an ongoing, retrospective, multicenter study aiming to enroll 5000 pts in 13 Sites from 10 European countries. Eligibility criteria include diagnosis of systemic AL amyloidosis, and first-line treatment initiation between 2004 and 2018. This report focuses on the most recent period and presents demographic and clinical characteristics as well as first- and second-line regimens for patients starting first line treatment between 2011 and 2018. Treatment information and efficacy outcomes for patients who participated in an interventional clinical trial have been excluded from the analysis.
Results
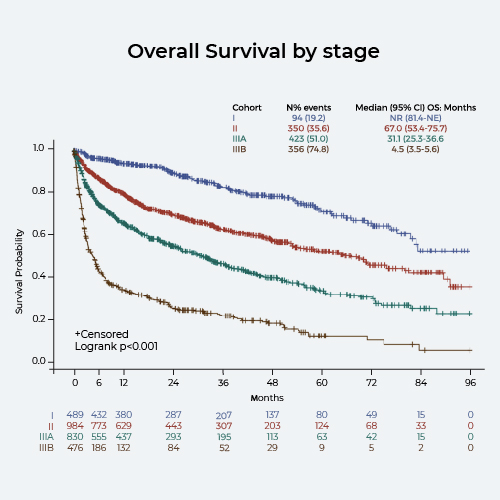
Overall, 3064 pts have been included from Austria (1.9%), Czech Republic (0.6%), France (5.8%), Germany (13.8%), Greece (5.8%), Italy (27.0%), the Netherlands (3.6%), Portugal (0.6%), Spain (2.9%), and UK (38.0%). The majority of pts were male (59%). Median age was 66 years, with 29% of the pts below 60 and 17% over 75 years. Heart (70%) and kidney (66%) were the organs most commonly involved and 17%, 35%, 28%, and 16% of the pts were at cardiac stage I, II, IIIA, and IIIB, respectively. Bortezomib-based (Bor-B) therapies were administered to 75% of pts at first line, without differences in age groups (74% in ages <65 years, 76% in ages ≥65), but they were more frequently used with more severe disease (65%, 73%, 81%, and 82% for pts of stage I, II, IIIA, and IIIB). Chemo-based regimens were administered to 9% of pts at first line, regardless of the cardiac stage but more frequently in older pts (5% vs 12%, for pts younger and older than 65, respectively). ASCT was used in 10% of those younger than 65, only in 2% those older than 65, and mostly to patients at earlier cardiac stage (14%, 4%, 3%, and 1% for stage I, II, IIIA, and IIIB). Overall, 984 pts received treatment at second line. Approximately half of the pts (48%) who received Bor-B therapy at first line received immunomodulatory-based (IMiD) at second line, 12% received chemo, 12% ASCT, and 14% were re-treated with Bor-B. About half of the pts (51%) who received chemo at first line continued with Bor-B at second line, 26% with IMiD, none received ASCT, and 3% proceeded with chemo again. Pts who received ASCT at first line continued with IMiD (40%), second ASCT (25%), Bor-B (8%), and chemo (4%). Median overall survival (OS) was 67, 31, and 4 months for pts of stages II, IIIA, and IIIB, and not reached for stage I pts (figure); for pts of revised stages (Mayo 2012) II, III, IV the median OS was 89.4, 29.6, and 14.3 months, and not reached for revised stage I pts.
Conclusion
Bortezomib-based regimens are the most commonly used in the period 2011-2018, across all patients groups, irrespectively of age or cardiac stage. ASCT and chemotherapy-based are used only in a minority of patients in Europe and seldom in patients with advanced cardiac disease. The dismal prognosis of stage IIIB pts even in recent years shows that there is an urgent medical need for more efficacious treatments and new strategies for these patients.
Keyword(s): AL amyloidosis
Abstract: EP1011
Type: E-Poster Presentation
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
Systemic light chain (AL) amyloidosis is a serious plasma cell disorder where amyloid is deposited in various organs and tissues throughout the body, disrupting their function, and severely affecting the quality of life of the patients (pts). Therapeutic regimens have been used off-label until very recently, therefore leading to differences in treatment patterns across Europe.
Aims
The current analysis aims to describe differences in patient characteristics and treatment strategies among different European countries in the recent years.
Methods
EMN23 is an ongoing, retrospective, multicenter study aiming to enroll 5000 pts in 13 Sites from 10 European countries. Eligibility criteria include diagnosis of systemic AL amyloidosis, and first-line treatment initiation between 2004 and 2018. This report focuses on the most recent period and presents demographic and clinical characteristics as well as first- and second-line regimens for patients starting first line treatment between 2011 and 2018. Treatment information and efficacy outcomes for patients who participated in an interventional clinical trial have been excluded from the analysis.
Results
Overall, 3064 pts have been included from Austria (1.9%), Czech Republic (0.6%), France (5.8%), Germany (13.8%), Greece (5.8%), Italy (27.0%), the Netherlands (3.6%), Portugal (0.6%), Spain (2.9%), and UK (38.0%). The majority of pts were male (59%). Median age was 66 years, with 29% of the pts below 60 and 17% over 75 years. Heart (70%) and kidney (66%) were the organs most commonly involved and 17%, 35%, 28%, and 16% of the pts were at cardiac stage I, II, IIIA, and IIIB, respectively. Bortezomib-based (Bor-B) therapies were administered to 75% of pts at first line, without differences in age groups (74% in ages <65 years, 76% in ages ≥65), but they were more frequently used with more severe disease (65%, 73%, 81%, and 82% for pts of stage I, II, IIIA, and IIIB). Chemo-based regimens were administered to 9% of pts at first line, regardless of the cardiac stage but more frequently in older pts (5% vs 12%, for pts younger and older than 65, respectively). ASCT was used in 10% of those younger than 65, only in 2% those older than 65, and mostly to patients at earlier cardiac stage (14%, 4%, 3%, and 1% for stage I, II, IIIA, and IIIB). Overall, 984 pts received treatment at second line. Approximately half of the pts (48%) who received Bor-B therapy at first line received immunomodulatory-based (IMiD) at second line, 12% received chemo, 12% ASCT, and 14% were re-treated with Bor-B. About half of the pts (51%) who received chemo at first line continued with Bor-B at second line, 26% with IMiD, none received ASCT, and 3% proceeded with chemo again. Pts who received ASCT at first line continued with IMiD (40%), second ASCT (25%), Bor-B (8%), and chemo (4%). Median overall survival (OS) was 67, 31, and 4 months for pts of stages II, IIIA, and IIIB, and not reached for stage I pts (figure); for pts of revised stages (Mayo 2012) II, III, IV the median OS was 89.4, 29.6, and 14.3 months, and not reached for revised stage I pts.

Conclusion
Bortezomib-based regimens are the most commonly used in the period 2011-2018, across all patients groups, irrespectively of age or cardiac stage. ASCT and chemotherapy-based are used only in a minority of patients in Europe and seldom in patients with advanced cardiac disease. The dismal prognosis of stage IIIB pts even in recent years shows that there is an urgent medical need for more efficacious treatments and new strategies for these patients.
Keyword(s): AL amyloidosis


