
Contributions
Abstract: EP1009
Type: E-Poster Presentation
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
Patients with relapsed and refractory multiple myeloma (RRMM) previously exposed to immunomodulatory agents, proteasome inhibitors (PIs), and anti-CD38 monoclonal antibodies (mAbs) have poor outcomes with subsequent treatments. In the pivotal KarMMa trial, ide-cel, a BCMA-directed CAR T cell therapy, showed frequent, deep, and durable responses in heavily pretreated patients with RRMM (Munshi NC, et al. N Engl J Med 2021;384:705-716).
Aims
To report updated efficacy and safety data from the phase 2 KarMMa trial (NCT03361748).
Methods
Eligible patients had received ≥ 3 prior regimens (including an immunomodulatory agent, a PI, and an anti-CD38 mAb) and were refractory to their last regimen per IMWG criteria. After 3 days of lymphodepletion (cyclophosphamide 300 mg/m2 + fludarabine 30 mg/m2), patients received 150─450 × 106 CAR+ T cells (target dose levels). The primary endpoint was overall response rate (ORR). The key secondary endpoints included complete response (CR) rate (CR + stringent CR [sCR]). Other secondary endpoints included duration of response (DOR), progression-free survival (PFS), overall survival (OS), and safety.
Results
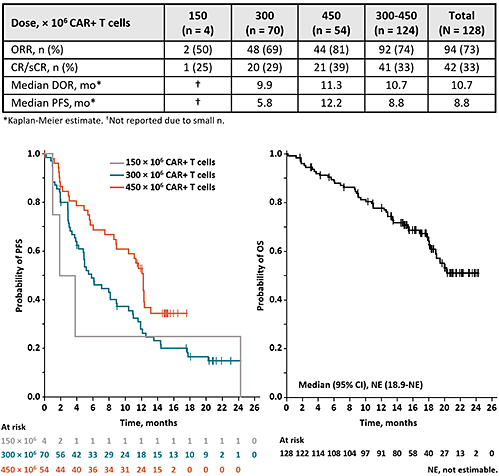
Of the 140 patients enrolled in KarMMa, 128 received ide-cel. Data are presented for the treated patients, who had a median age of 61 years and had received a median of 6 (range, 3-16) prior regimens; 84% were triple-class refractory, and 26% were penta-refractory (lenalidomide, pomalidomide, bortezomib, carfilzomib, and daratumumab). Most patients (88%) had received bridging therapy. At the data cutoff (7 April 2020), the median follow-up was 15.4 months. The ORR was 73% and the median PFS was 8.8 months; both increased with higher dose (Table). At the highest target dose (450 × 106 CAR+ T cells), the ORR was 81%, the CR rate was 39%, and the median PFS increased to 12.2 months with longer follow-up. Responses were observed in all subgroups, including difficult-to-treat subsets (eg, high tumor burden [ORR, 71%], extramedullary disease [70%], and R-ISS stage III disease [48%]). OS continues to mature, and the median has not been reached (Figure); the estimated 15-month OS rate was 71%. The most common any-grade toxicities were cytopenia (97%) and cytokine release syndrome (CRS; 84%). CRS was mostly grade 1/2; 5 patients (4%) had grade 3, 1 had grade 4 (at 300 × 106), and 1 had grade 5 (at 300 × 106) events. Investigator-identified neurotoxicity was reported in 23 patients (18%); 4 patients (3%) had grade 3 and 0 had grade ≥ 4 events. Tocilizumab was used in 67 and 3 patients with CRS and neurotoxicity, respectively. Similarly, steroids were used in 19 and 10 patients with CRS and neurotoxicity, respectively.
Conclusion
Updated results from the KarMMa trial continue to demonstrate deep, durable responses with ide-cel in heavily pretreated, triple-class–exposed patients with RRMM. Efficacy and safety reflect prior reports and support a favorable clinical benefit-risk profile for ide-cel across the target dose levels.
Keyword(s): CAR-T, Myeloma, Refractory, Relapse
Abstract: EP1009
Type: E-Poster Presentation
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
Patients with relapsed and refractory multiple myeloma (RRMM) previously exposed to immunomodulatory agents, proteasome inhibitors (PIs), and anti-CD38 monoclonal antibodies (mAbs) have poor outcomes with subsequent treatments. In the pivotal KarMMa trial, ide-cel, a BCMA-directed CAR T cell therapy, showed frequent, deep, and durable responses in heavily pretreated patients with RRMM (Munshi NC, et al. N Engl J Med 2021;384:705-716).
Aims
To report updated efficacy and safety data from the phase 2 KarMMa trial (NCT03361748).
Methods
Eligible patients had received ≥ 3 prior regimens (including an immunomodulatory agent, a PI, and an anti-CD38 mAb) and were refractory to their last regimen per IMWG criteria. After 3 days of lymphodepletion (cyclophosphamide 300 mg/m2 + fludarabine 30 mg/m2), patients received 150─450 × 106 CAR+ T cells (target dose levels). The primary endpoint was overall response rate (ORR). The key secondary endpoints included complete response (CR) rate (CR + stringent CR [sCR]). Other secondary endpoints included duration of response (DOR), progression-free survival (PFS), overall survival (OS), and safety.
Results
Of the 140 patients enrolled in KarMMa, 128 received ide-cel. Data are presented for the treated patients, who had a median age of 61 years and had received a median of 6 (range, 3-16) prior regimens; 84% were triple-class refractory, and 26% were penta-refractory (lenalidomide, pomalidomide, bortezomib, carfilzomib, and daratumumab). Most patients (88%) had received bridging therapy. At the data cutoff (7 April 2020), the median follow-up was 15.4 months. The ORR was 73% and the median PFS was 8.8 months; both increased with higher dose (Table). At the highest target dose (450 × 106 CAR+ T cells), the ORR was 81%, the CR rate was 39%, and the median PFS increased to 12.2 months with longer follow-up. Responses were observed in all subgroups, including difficult-to-treat subsets (eg, high tumor burden [ORR, 71%], extramedullary disease [70%], and R-ISS stage III disease [48%]). OS continues to mature, and the median has not been reached (Figure); the estimated 15-month OS rate was 71%. The most common any-grade toxicities were cytopenia (97%) and cytokine release syndrome (CRS; 84%). CRS was mostly grade 1/2; 5 patients (4%) had grade 3, 1 had grade 4 (at 300 × 106), and 1 had grade 5 (at 300 × 106) events. Investigator-identified neurotoxicity was reported in 23 patients (18%); 4 patients (3%) had grade 3 and 0 had grade ≥ 4 events. Tocilizumab was used in 67 and 3 patients with CRS and neurotoxicity, respectively. Similarly, steroids were used in 19 and 10 patients with CRS and neurotoxicity, respectively.

Conclusion
Updated results from the KarMMa trial continue to demonstrate deep, durable responses with ide-cel in heavily pretreated, triple-class–exposed patients with RRMM. Efficacy and safety reflect prior reports and support a favorable clinical benefit-risk profile for ide-cel across the target dose levels.
Keyword(s): CAR-T, Myeloma, Refractory, Relapse


