
Contributions
Abstract: S312
Type: Oral Presentation
Session title: Patient's voice and health outcomes in hematology
Background
Cold agglutinin disease (CAD) is a rare autoimmune hemolytic anemia characterized by classical complement pathway (CP)–mediated hemolysis. Apart from anemia, patients with CAD experience fatigue and poor quality of life (QOL). Before the CARDINAL study, QOL and patient-reported outcomes (PRO) such as Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue were not examined in CAD patients. Sutimlimab (formerly BIVV009), a humanized monoclonal antibody that selectively inhibits C1s of the C1 complex, prevents classical CP activation, while leaving alternative and lectin pathways intact. In CARDINAL Part A, sutimlimab treatment resulted in rapid, clinically meaningful improvement in all PRO measures (Röth et al. ASH 2020), in addition to halting hemolysis, improving hemoglobin (Hb) levels, and normalizing bilirubin levels, in patients with CAD (Röth et al. ASH 2019).
Aims
To report sutimlimab effect on PROs at 1-year, from the ongoing CARDINAL Part B extension (data cut: January 16, 2020).
Methods
CARDINAL is a Phase 3, open-label, single-arm, multicenter, two-part study. After informed consent and study enrollment, sutimlimab was administered on Days 0 and 7, followed by biweekly infusions, in both Parts A and B. Interim Part B efficacy endpoints included evaluation of Hb levels and other hemolytic markers over 1-year and QOL assessed by the FACIT-Fatigue Scale, to Week 51 (last scheduled visit within 1-year of treatment), in patients with CAD. Mean change from baseline for exploratory QOL endpoints such as EuroQol 5-dimension 5-level (EQ-5D-5L) scores, 12-Item Short Form Health Survey (SF-12), Patient Global Impression of Change (PGIC), and Patient Global Impression of (fatigue) Severity (PGIS) were also assessed at Week 51. Outcomes were reported using descriptive statistics.
Results
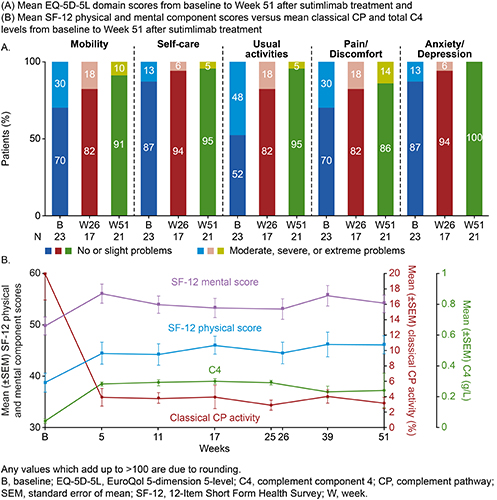
Of 24 enrolled patients, 22 patients completed Part A, and all entered Part B. At baseline, mean (standard deviation [SD]) FACIT-Fatigue score was 32.5 (10.6) points and mean (SD) EQ-5D-5L index and visual analog scale (VAS) scores were 0.70 (0.23) and 62.0 (14.7), respectively. Mean (SD) SF-12 physical and mental component scores were 38.7 (8.7) and 49.8 (8.2). One-third of patients (33%) had severe PGIS fatigue status at baseline. Upon sutimlimab treatment, mean (SD) FACIT-Fatigue scores improved within 1-week (n=22/24), with scores >40 from Week 3 (Part A) to Week 51. Overall, from baseline to Week 51, mean (SD) FACIT-Fatigue score (n=18/22) was improved by 11.4 (11.9) points. The mean (SD) change from baseline in EQ-5D-5L index and VAS scores (n=20/23) at Week 51 was 0.08 (0.19) and 14.50 (19.05). At Week 51, among 21 evaluable patients, none reported anxiety/depression; considerable improvements were seen in mobility, self-care, and usual activities domains of the EQ-5D-5L (Figure A). The mean (SD) increase in SF-12 physical and mental component scores (n=19/22) from baseline to Week 51 were 6.6 (9.7) and 3.6 (9.4). SF-12 score increases appear to coincide with reduction in classical CP activity and normalized total C4 levels (Figure B). At Week 51, among 21 evaluable patients, a majority (90.5%) indicated “improved” disease state on the PGIC scale; no patients reported severe fatigue on completing PGIS, compared with one-third at baseline (n=2/6).
Conclusion
One-year interim follow-up CARDINAL study results demonstrate that continuous classical CP inhibition with sutimlimab resulted in rapid, sustained improvements in all PRO measures evaluated, further demonstrating the meaningful impact of sutimlimab on patient QOL.
Keyword(s): Autoimmune hemolytic anemia (AIHA), Complement, Phase III
Abstract: S312
Type: Oral Presentation
Session title: Patient's voice and health outcomes in hematology
Background
Cold agglutinin disease (CAD) is a rare autoimmune hemolytic anemia characterized by classical complement pathway (CP)–mediated hemolysis. Apart from anemia, patients with CAD experience fatigue and poor quality of life (QOL). Before the CARDINAL study, QOL and patient-reported outcomes (PRO) such as Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue were not examined in CAD patients. Sutimlimab (formerly BIVV009), a humanized monoclonal antibody that selectively inhibits C1s of the C1 complex, prevents classical CP activation, while leaving alternative and lectin pathways intact. In CARDINAL Part A, sutimlimab treatment resulted in rapid, clinically meaningful improvement in all PRO measures (Röth et al. ASH 2020), in addition to halting hemolysis, improving hemoglobin (Hb) levels, and normalizing bilirubin levels, in patients with CAD (Röth et al. ASH 2019).
Aims
To report sutimlimab effect on PROs at 1-year, from the ongoing CARDINAL Part B extension (data cut: January 16, 2020).
Methods
CARDINAL is a Phase 3, open-label, single-arm, multicenter, two-part study. After informed consent and study enrollment, sutimlimab was administered on Days 0 and 7, followed by biweekly infusions, in both Parts A and B. Interim Part B efficacy endpoints included evaluation of Hb levels and other hemolytic markers over 1-year and QOL assessed by the FACIT-Fatigue Scale, to Week 51 (last scheduled visit within 1-year of treatment), in patients with CAD. Mean change from baseline for exploratory QOL endpoints such as EuroQol 5-dimension 5-level (EQ-5D-5L) scores, 12-Item Short Form Health Survey (SF-12), Patient Global Impression of Change (PGIC), and Patient Global Impression of (fatigue) Severity (PGIS) were also assessed at Week 51. Outcomes were reported using descriptive statistics.
Results
Of 24 enrolled patients, 22 patients completed Part A, and all entered Part B. At baseline, mean (standard deviation [SD]) FACIT-Fatigue score was 32.5 (10.6) points and mean (SD) EQ-5D-5L index and visual analog scale (VAS) scores were 0.70 (0.23) and 62.0 (14.7), respectively. Mean (SD) SF-12 physical and mental component scores were 38.7 (8.7) and 49.8 (8.2). One-third of patients (33%) had severe PGIS fatigue status at baseline. Upon sutimlimab treatment, mean (SD) FACIT-Fatigue scores improved within 1-week (n=22/24), with scores >40 from Week 3 (Part A) to Week 51. Overall, from baseline to Week 51, mean (SD) FACIT-Fatigue score (n=18/22) was improved by 11.4 (11.9) points. The mean (SD) change from baseline in EQ-5D-5L index and VAS scores (n=20/23) at Week 51 was 0.08 (0.19) and 14.50 (19.05). At Week 51, among 21 evaluable patients, none reported anxiety/depression; considerable improvements were seen in mobility, self-care, and usual activities domains of the EQ-5D-5L (Figure A). The mean (SD) increase in SF-12 physical and mental component scores (n=19/22) from baseline to Week 51 were 6.6 (9.7) and 3.6 (9.4). SF-12 score increases appear to coincide with reduction in classical CP activity and normalized total C4 levels (Figure B). At Week 51, among 21 evaluable patients, a majority (90.5%) indicated “improved” disease state on the PGIC scale; no patients reported severe fatigue on completing PGIS, compared with one-third at baseline (n=2/6).

Conclusion
One-year interim follow-up CARDINAL study results demonstrate that continuous classical CP inhibition with sutimlimab resulted in rapid, sustained improvements in all PRO measures evaluated, further demonstrating the meaningful impact of sutimlimab on patient QOL.
Keyword(s): Autoimmune hemolytic anemia (AIHA), Complement, Phase III


