
Contributions
Abstract: S311
Type: Oral Presentation
Session title: Patient's voice and health outcomes in hematology
Background
While ~50% of patients (pts) with AML aged >60 years attain complete remission (CR) with intensive chemotherapy (IC), up to 90% who do will eventually relapse. In the continuum of AML care, the highest costs are related to relapsed/refractory disease, and hospitalization is the largest component (~70%) of direct healthcare costs. In the randomized, phase 3 QUAZAR AML-001 trial, maintenance treatment (Tx) with Oral-AZA significantly prolonged overall and relapse-free survival vs. PBO for pts with AML in first remission after IC.
Aims
Determine rates of hospitalization and days (d) in hospital with Oral-AZA and PBO for all pts in QUAZAR AML-001, and estimate associated hospitalization costs in Spain from the Spanish National Health System (NHS) perspective, and in the UK from the UK NHS perspective.
Methods
Eligible pts were age ≥55 yrs, had intermediate- or poor-risk cytogenetics and ECOG PS ≤3, and were not candidates for transplant. Within 4 months (mo) of achieving CR or CR with incomplete blood count recovery (CRi) after induction ± consolidation, pts were randomized 1:1 to Oral-AZA 300-mg or PBO QD for 14d/28d cycle. Pts who received ≥1 study drug dose were followed for hospitalization from informed consent through 28d after last dose. The mean number of hospital days/mo (30d) was the total number of days in hospital divided by the number of ongoing pts each mo. Hospitalization rates and durations were adjusted for total drug exposure. Confidence intervals (CI) for relative risk (RR) estimates and related P values are based on asymptotic methods.
Unit cost of hospitalization in Spain was derived from literature (Arenaza et al. Clinicoecon Outcomes Res, 2019), and in the UK from NHS reference costs (https://improvement.nhs.uk/resources/reference-costs/#rc1718), as the average total AML-related hospitalization costs per day, adjusted for inflation to 2019 prices, using the medical component of the consumer price index for each country.
Results
469 pts received Oral-AZA (n=236) or PBO (n=233); median time on Tx was 11.6 vs 5.7 mo, respectively, and total exposures were 363.8 pt-years (PY) and 234.9 PY. Similar proportions of patients were hospitalized during the study: Oral-AZA 46% (n=108), PBO 51% (n=118). Total number of hospitalization events was numerically higher in the Oral-AZA arm (173 vs 151 in the PBO arm), but the exposure-adjusted hospitalization rate was significantly lower with Oral-AZA: 0.48/PY vs. 0.64/PY (RR 0.740 [95%CI 0.595, 0.920]; P=0.0068). Total days hospitalized were 2,872 in the Oral-AZA arm and 3,139 in the PBO arm; the exposure-adjusted rate was also significantly lower with Oral-AZA (7.89 vs. 13.36 d/PY, respectively; RR 0.591 [95%CI 0.562, 0.621]; P<0.0001).
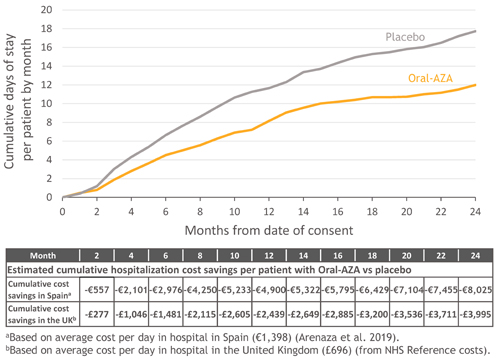
Mean hospitalization cost in Spain was €1,398/d. Using exposure-adjusted days-in-hospital, estimated mean costs of hospitalization in Spain were €11,030/PY with Oral-AZA and €18,676/PY with PBO. Cumulative cost savings with Oral-AZA vs. PBO ranged from €557 at mo 2 to €8,025 by mo 24 (Figure).
In the UK, mean hospitalization cost was £696/d, resulting in estimated exposure-adjusted mean costs of £5,490/PY with Oral-AZA and £9,296/PY with PBO. Cumulative cost savings with Oral-AZA in the UK ranged from £277 at mo 2 to £3,995 by mo 24 (Figure).
Conclusion
Compared with PBO, Oral-AZA was associated with significantly reduced exposure-adjusted rates of hospitalization and days in hospital, and considerable estimated cost savings in Spain and the UK. Delaying relapse with Oral-AZA may lead to substantial economic benefits due to lower hospitalization costs.
Keyword(s): AML, Azacitidine, Cost analysis, Maintenance
Abstract: S311
Type: Oral Presentation
Session title: Patient's voice and health outcomes in hematology
Background
While ~50% of patients (pts) with AML aged >60 years attain complete remission (CR) with intensive chemotherapy (IC), up to 90% who do will eventually relapse. In the continuum of AML care, the highest costs are related to relapsed/refractory disease, and hospitalization is the largest component (~70%) of direct healthcare costs. In the randomized, phase 3 QUAZAR AML-001 trial, maintenance treatment (Tx) with Oral-AZA significantly prolonged overall and relapse-free survival vs. PBO for pts with AML in first remission after IC.
Aims
Determine rates of hospitalization and days (d) in hospital with Oral-AZA and PBO for all pts in QUAZAR AML-001, and estimate associated hospitalization costs in Spain from the Spanish National Health System (NHS) perspective, and in the UK from the UK NHS perspective.
Methods
Eligible pts were age ≥55 yrs, had intermediate- or poor-risk cytogenetics and ECOG PS ≤3, and were not candidates for transplant. Within 4 months (mo) of achieving CR or CR with incomplete blood count recovery (CRi) after induction ± consolidation, pts were randomized 1:1 to Oral-AZA 300-mg or PBO QD for 14d/28d cycle. Pts who received ≥1 study drug dose were followed for hospitalization from informed consent through 28d after last dose. The mean number of hospital days/mo (30d) was the total number of days in hospital divided by the number of ongoing pts each mo. Hospitalization rates and durations were adjusted for total drug exposure. Confidence intervals (CI) for relative risk (RR) estimates and related P values are based on asymptotic methods.
Unit cost of hospitalization in Spain was derived from literature (Arenaza et al. Clinicoecon Outcomes Res, 2019), and in the UK from NHS reference costs (https://improvement.nhs.uk/resources/reference-costs/#rc1718), as the average total AML-related hospitalization costs per day, adjusted for inflation to 2019 prices, using the medical component of the consumer price index for each country.
Results
469 pts received Oral-AZA (n=236) or PBO (n=233); median time on Tx was 11.6 vs 5.7 mo, respectively, and total exposures were 363.8 pt-years (PY) and 234.9 PY. Similar proportions of patients were hospitalized during the study: Oral-AZA 46% (n=108), PBO 51% (n=118). Total number of hospitalization events was numerically higher in the Oral-AZA arm (173 vs 151 in the PBO arm), but the exposure-adjusted hospitalization rate was significantly lower with Oral-AZA: 0.48/PY vs. 0.64/PY (RR 0.740 [95%CI 0.595, 0.920]; P=0.0068). Total days hospitalized were 2,872 in the Oral-AZA arm and 3,139 in the PBO arm; the exposure-adjusted rate was also significantly lower with Oral-AZA (7.89 vs. 13.36 d/PY, respectively; RR 0.591 [95%CI 0.562, 0.621]; P<0.0001).
Mean hospitalization cost in Spain was €1,398/d. Using exposure-adjusted days-in-hospital, estimated mean costs of hospitalization in Spain were €11,030/PY with Oral-AZA and €18,676/PY with PBO. Cumulative cost savings with Oral-AZA vs. PBO ranged from €557 at mo 2 to €8,025 by mo 24 (Figure).
In the UK, mean hospitalization cost was £696/d, resulting in estimated exposure-adjusted mean costs of £5,490/PY with Oral-AZA and £9,296/PY with PBO. Cumulative cost savings with Oral-AZA in the UK ranged from £277 at mo 2 to £3,995 by mo 24 (Figure).

Conclusion
Compared with PBO, Oral-AZA was associated with significantly reduced exposure-adjusted rates of hospitalization and days in hospital, and considerable estimated cost savings in Spain and the UK. Delaying relapse with Oral-AZA may lead to substantial economic benefits due to lower hospitalization costs.
Keyword(s): AML, Azacitidine, Cost analysis, Maintenance


