
Contributions
Abstract: S306
Type: Oral Presentation
Session title: Malignancies and thrombosis
Background
Venous thromboembolism (VTE) is one serious complication in patients with acute lymphoblastic leukemia (ALL) with an incidence of 1-14% in thromboembolic symptomatic patients and up to 37% in the asymptomatic. In these patients, VTE is a multifactorial event: leukemia, central venous catheter (CVC), chemotherapy, in particular, asparaginase (ASP), and steroids. ASP is an effective chemotherapeutic agent that catalyzes the hydrolysis of asparagine. Treatment with ASP increases the risk for VTE. Among the causes that can determine an increased thromboembolic risk, the presence of congenital or acquired thrombophilia can play a crucial role.
Aims
The aim of our study was to evaluate the role of thrombophilia on VTE events in pediatric patients (age < 18 years) and in adolescents and young adults (AYA) < 40 years old with ALL during ASP chemotherapy regimens.
Methods
We defined ASP-associated thrombosis when the event occurred within 1 month from the drug administration. The types of drugs used were: the native form derived from E. Coli (Medac® and Kidrolase®); the form derived from Erwinia Chrysanthemi (Erwinase®); and the pegylated E. coli ASP (PEG-ASP -Oncaspar®). Patients were treated according to the following chemotherapy protocols: GIMEMA LAL 1913; GIMEMA LAL 1308; GIMEMA LAL 2000; NILG-LAL 10/07; GIMEMA LAL 0904; GIMEMA LAL 0496; ESPHALL; AIEOP-EURO-LB02; AIEOP-BFM LAL2009; AIEOP-BFM LAL2000. All patients performed the inherited and acquired thrombophilia assays at disease diagnosis before chemotherapy start. In patients with clinical signs of thrombotic occlusion, the diagnosis of VTE was made with Doppler ultrasonography examination of vessels; chest computerized tomography (CT) for pulmonary embolism; magnetic resonance imaging (MRI) in case of thrombosis of the cerebral circulation.
Results
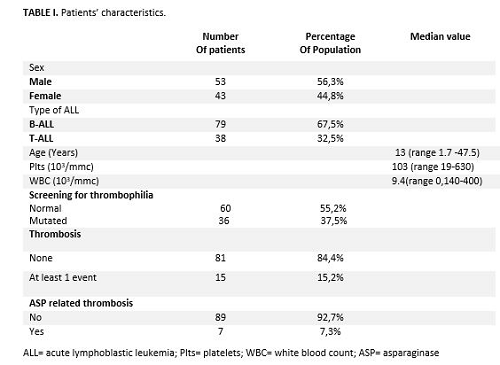
We studied 96 ALL patients and their characteristics are summarized in table 1. Thrombotic complications occurred in 15/96 (15.6%) patients, including 12 deep vein thromboses (DVT) of upper limbs, 1 thrombosis of the central vein of retina, 1 thrombosis of the superior sagittal sinus, 1 DVT extended to both lower and upper limbs associated with pulmonary embolism. Overall, 6/15 (40%) patients with the thrombotic complication presented thrombophilia (p = 0.265). Of the 15 thrombotic events, 7/15 (47%) were associated with the use of ASP. Three/seven (43%) patients presented B-ALL and 4/7 (57%) T-ALL (p = 0.027). Five/7 were treated with PEG-ASP (Oncaspar®) and 2/7 with the native E.coli ASP (p = 0.511); notably, 5 were thrombophilic (p=0.008). In the multivariate analysis, among the predictive factors for ASP-related thrombosis considered (sex, age, thrombophilia, and T-ALL), the only significant factor for thrombotic events development was thrombophilia (p = 0.0029, IC 0.025-0.823; HR 0.143).
Conclusion
Although thrombophilia is a well-known risk factor for VTE in ALL, our study focused on its link with ASP-related VTE. We suggests that screening for thrombophilia should be performed in ALL patients before ASP administration, since thrombophilic ALL patients may benefit from VTE prophylaxis.
Keyword(s): Acute lymphoblastic leukemia, Asparaginase, Thrombophilia
Abstract: S306
Type: Oral Presentation
Session title: Malignancies and thrombosis
Background
Venous thromboembolism (VTE) is one serious complication in patients with acute lymphoblastic leukemia (ALL) with an incidence of 1-14% in thromboembolic symptomatic patients and up to 37% in the asymptomatic. In these patients, VTE is a multifactorial event: leukemia, central venous catheter (CVC), chemotherapy, in particular, asparaginase (ASP), and steroids. ASP is an effective chemotherapeutic agent that catalyzes the hydrolysis of asparagine. Treatment with ASP increases the risk for VTE. Among the causes that can determine an increased thromboembolic risk, the presence of congenital or acquired thrombophilia can play a crucial role.
Aims
The aim of our study was to evaluate the role of thrombophilia on VTE events in pediatric patients (age < 18 years) and in adolescents and young adults (AYA) < 40 years old with ALL during ASP chemotherapy regimens.
Methods
We defined ASP-associated thrombosis when the event occurred within 1 month from the drug administration. The types of drugs used were: the native form derived from E. Coli (Medac® and Kidrolase®); the form derived from Erwinia Chrysanthemi (Erwinase®); and the pegylated E. coli ASP (PEG-ASP -Oncaspar®). Patients were treated according to the following chemotherapy protocols: GIMEMA LAL 1913; GIMEMA LAL 1308; GIMEMA LAL 2000; NILG-LAL 10/07; GIMEMA LAL 0904; GIMEMA LAL 0496; ESPHALL; AIEOP-EURO-LB02; AIEOP-BFM LAL2009; AIEOP-BFM LAL2000. All patients performed the inherited and acquired thrombophilia assays at disease diagnosis before chemotherapy start. In patients with clinical signs of thrombotic occlusion, the diagnosis of VTE was made with Doppler ultrasonography examination of vessels; chest computerized tomography (CT) for pulmonary embolism; magnetic resonance imaging (MRI) in case of thrombosis of the cerebral circulation.
Results
We studied 96 ALL patients and their characteristics are summarized in table 1. Thrombotic complications occurred in 15/96 (15.6%) patients, including 12 deep vein thromboses (DVT) of upper limbs, 1 thrombosis of the central vein of retina, 1 thrombosis of the superior sagittal sinus, 1 DVT extended to both lower and upper limbs associated with pulmonary embolism. Overall, 6/15 (40%) patients with the thrombotic complication presented thrombophilia (p = 0.265). Of the 15 thrombotic events, 7/15 (47%) were associated with the use of ASP. Three/seven (43%) patients presented B-ALL and 4/7 (57%) T-ALL (p = 0.027). Five/7 were treated with PEG-ASP (Oncaspar®) and 2/7 with the native E.coli ASP (p = 0.511); notably, 5 were thrombophilic (p=0.008). In the multivariate analysis, among the predictive factors for ASP-related thrombosis considered (sex, age, thrombophilia, and T-ALL), the only significant factor for thrombotic events development was thrombophilia (p = 0.0029, IC 0.025-0.823; HR 0.143).

Conclusion
Although thrombophilia is a well-known risk factor for VTE in ALL, our study focused on its link with ASP-related VTE. We suggests that screening for thrombophilia should be performed in ALL patients before ASP administration, since thrombophilic ALL patients may benefit from VTE prophylaxis.
Keyword(s): Acute lymphoblastic leukemia, Asparaginase, Thrombophilia


