
Contributions
Abstract: S305
Type: Oral Presentation
Session title: Malignancies and thrombosis
Background
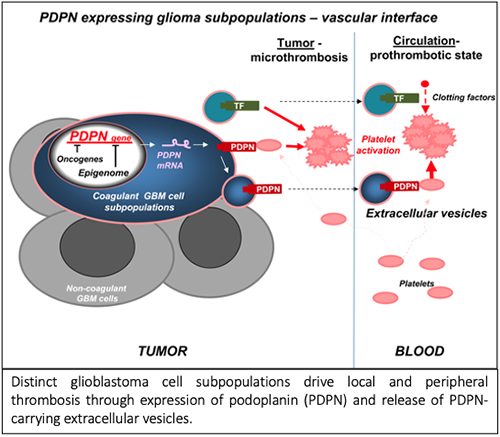
Vascular anomalies, including thrombosis, are a hallmark of glioblastoma (GBM) and an aftermath of dysregulated cancer cell genome and epigenome. The upregulation of podoplanin (PDPN) by cancer cells has recently been linked to an increased risk of venous thrombo-embolism (VTE) in GBM patients. Thus, regulation of this platelet activating protein by transforming events and release from cancer cells is of considerable interest.
Aims
I. (a) Investigate the pattern of PDPN expression and characterize PDPN expressing populations. (b) Evaluate the contribution of oncogenic driver pathways to PDPN expression modulation in GBM models. (c) Explore the modes of PDPN expression modulation by implicated oncogenic drivers.
II. (a) Investigate the potential involvement of extracellular vesicles as route for systemic dissemination of GBM-associated PDPN. (b) Examine the role of GBM-associated and systemically disseminated PDPN in peripheral activation of platelets. (c) Assess the contribution of PDPN to intratumoral thrombosis and explore the possible cooperation with TF.
Methods
We used single cell and bulk transcriptome data mining, as well as cellular and xenograft models in mice to analyze the nature of cells expressing PDPN, their impact on activation of coagulation system, and platelets. We also took advantage of various analytical techniques including immunoblotting, immunofluorescence, electron microscopy, density gradient fractionation, Cytoflex, and ELISA.
Results
We report that PDPN is expressed by distinct (mesenchymal) GBM cell subpopulation and downregulated by oncogenic mutations of EGFR and IDH1 genes, via changes in chromatin modifications (EZH2) and DNA methylation, respectively. GBM cells exteriorize their PDPN and/or tissue factor (TF) as cargo of exosome-like extracellular vesicles (EVs) shed from cells in vitro and in vivo. Injection of glioma PDPN-EVs activates platelets, while TF-EVs weakly activate the clotting cascade. Similarly, increase of platelet activation (PF4) or coagulation markers (D-dimer) occurs in mice harboring the corresponding glioma xenografts expressing PDPN or TF, respectively. Co-expression of PDPN and TF by GBM cells cooperatively affects tumor microthrombosis.
Conclusion
In GBM distinct cellular subsets drive multiple facets of cancer-associated thrombosis and may represent targets for phenotype and cell type-based diagnosis and antithrombotic intervention. Accordingly, we suggest that the preponderance of PDPN expression as a risk factor in GBM and involvement of platelets in our GBM model may merit investigating antiplatelets for potential inclusion in VTE management strategies in GBM setting.
Keyword(s): Cancer, Coagulopathy, Oncogene, Thrombosis
Abstract: S305
Type: Oral Presentation
Session title: Malignancies and thrombosis
Background
Vascular anomalies, including thrombosis, are a hallmark of glioblastoma (GBM) and an aftermath of dysregulated cancer cell genome and epigenome. The upregulation of podoplanin (PDPN) by cancer cells has recently been linked to an increased risk of venous thrombo-embolism (VTE) in GBM patients. Thus, regulation of this platelet activating protein by transforming events and release from cancer cells is of considerable interest.
Aims
I. (a) Investigate the pattern of PDPN expression and characterize PDPN expressing populations. (b) Evaluate the contribution of oncogenic driver pathways to PDPN expression modulation in GBM models. (c) Explore the modes of PDPN expression modulation by implicated oncogenic drivers.
II. (a) Investigate the potential involvement of extracellular vesicles as route for systemic dissemination of GBM-associated PDPN. (b) Examine the role of GBM-associated and systemically disseminated PDPN in peripheral activation of platelets. (c) Assess the contribution of PDPN to intratumoral thrombosis and explore the possible cooperation with TF.
Methods
We used single cell and bulk transcriptome data mining, as well as cellular and xenograft models in mice to analyze the nature of cells expressing PDPN, their impact on activation of coagulation system, and platelets. We also took advantage of various analytical techniques including immunoblotting, immunofluorescence, electron microscopy, density gradient fractionation, Cytoflex, and ELISA.
Results
We report that PDPN is expressed by distinct (mesenchymal) GBM cell subpopulation and downregulated by oncogenic mutations of EGFR and IDH1 genes, via changes in chromatin modifications (EZH2) and DNA methylation, respectively. GBM cells exteriorize their PDPN and/or tissue factor (TF) as cargo of exosome-like extracellular vesicles (EVs) shed from cells in vitro and in vivo. Injection of glioma PDPN-EVs activates platelets, while TF-EVs weakly activate the clotting cascade. Similarly, increase of platelet activation (PF4) or coagulation markers (D-dimer) occurs in mice harboring the corresponding glioma xenografts expressing PDPN or TF, respectively. Co-expression of PDPN and TF by GBM cells cooperatively affects tumor microthrombosis.

Conclusion
In GBM distinct cellular subsets drive multiple facets of cancer-associated thrombosis and may represent targets for phenotype and cell type-based diagnosis and antithrombotic intervention. Accordingly, we suggest that the preponderance of PDPN expression as a risk factor in GBM and involvement of platelets in our GBM model may merit investigating antiplatelets for potential inclusion in VTE management strategies in GBM setting.
Keyword(s): Cancer, Coagulopathy, Oncogene, Thrombosis


