
Contributions
Abstract: S299
Type: Oral Presentation
Session title: ITP: from bench to bedside
Background
Immune thrombocytopenia (ITP) is characterized by immune-mediated platelet destruction and impaired production, triggering thrombocytopenia, and a predisposition to bleeding. Despite recent therapeutic advances, durable remission remains an unmet need for relapsed/refractory ITP patients. Rilzabrutinib is an oral, reversible, covalent inhibitor of BTK that targets immune-mediated ITP activities and has simultaneous rapid anti-inflammatory effects and neutralization and prevention of autoantibody signaling preclinically. The ongoing phase I/II study (NCT03395210) assesses the safety and efficacy of rilzabrutinib in patients with relapsed or refractory ITP who previously responded to ≥1 prior ITP therapy and have no available treatment options. Initial results have shown rapid and durable clinical activity and a well-tolerated safety profile in patients responding to rilzabrutinib.
Aims
Phase II evaluation of rilzabrutinib in the overall study population and patients initiating 400 mg BID rilzabrutinib.
Methods
Eligible patients with 2 baseline platelet counts <30×109/L received oral rilzabrutinib at starting doses of 200 mg QD, 400 mg QD, 300 mg BID, or 400 mg BID; intrapatient dose escalation was allowed to improve efficacy. Rilzabrutinib was given for 24 weeks; additional long-term therapy was administered at the 400 mg BID dose in responders only. Stable doses of concomitant ITP medication (corticosteroids and thrombopoietin-receptor agonists) were permitted for patients without adequate platelet response. The primary endpoints are safety and ≥2 consecutive platelet counts ≥50×109/L and increased ≥20×109/L from baseline without requiring rescue medication. All patients provided informed consent.
Results
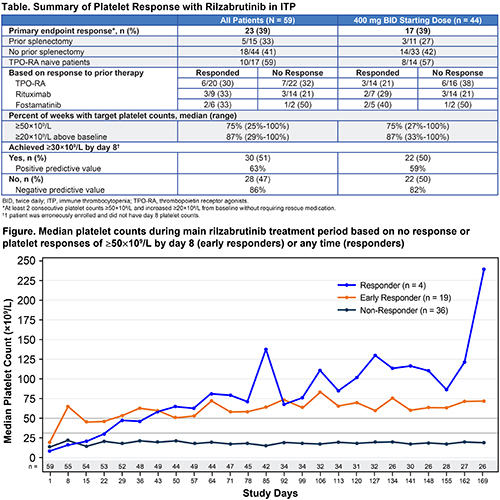
The overall study population comprises 59 patients, of whom 44 had a starting dose of 400 mg BID; data cutoff was 09Nov2020. Overall, patients had a median age 50 years (range, 21-74), median duration of ITP 6.4 years (range, 0.4-52.5), and a median of 6.0 prior therapies (range, 1-53; 25% prior splenectomy). Baseline median platelet counts were 15-16×109/L (range, 2-33). Baseline demographics were similar for patients initiating 400 mg BID. A total of 23 patients (39%) overall and 17 (39%) initiating 400 mg BID achieved the primary efficacy endpoint (Table). Primary responses were observed irrespective of prior treatments or responses. Responders maintained platelet counts ≥50×109/L for a median of 75% of weeks, as well as increased platelet counts of ≥20×109/L above baseline levels for a median of 87% of weeks (Figure). Approximately half of all patients attained platelet counts of ≥30×109/L by day 8. Day 8 platelet count was associated with a high negative predictive value for achieving the primary endpoint (>80%) and may assist with identifying patients unlikely to reach the targeted primary platelet response. Related treatment-emergent adverse events (all grade 1/2) were observed in 29 of 59 (49%) patients overall; most common were 31% diarrhea and 25% nausea (all others were <10%). There were no related bleeding/thrombotic events, ECG abnormalities, hypertension, or liver enzyme elevations.
Conclusion
Rilzabrutinib was well-tolerated and showed rapid and durable responses, with half of the patients achieving platelet counts ≥30×109/L by day 8, potentially providing a tool for predicting early response to treatment, and a durable primary endpoint response. Taken together, these findings support further investigation of the magnitude and durability of rilzabrutinib clinical benefit.
Keyword(s): Immune thrombocytopenia (ITP), Platelet
Abstract: S299
Type: Oral Presentation
Session title: ITP: from bench to bedside
Background
Immune thrombocytopenia (ITP) is characterized by immune-mediated platelet destruction and impaired production, triggering thrombocytopenia, and a predisposition to bleeding. Despite recent therapeutic advances, durable remission remains an unmet need for relapsed/refractory ITP patients. Rilzabrutinib is an oral, reversible, covalent inhibitor of BTK that targets immune-mediated ITP activities and has simultaneous rapid anti-inflammatory effects and neutralization and prevention of autoantibody signaling preclinically. The ongoing phase I/II study (NCT03395210) assesses the safety and efficacy of rilzabrutinib in patients with relapsed or refractory ITP who previously responded to ≥1 prior ITP therapy and have no available treatment options. Initial results have shown rapid and durable clinical activity and a well-tolerated safety profile in patients responding to rilzabrutinib.
Aims
Phase II evaluation of rilzabrutinib in the overall study population and patients initiating 400 mg BID rilzabrutinib.
Methods
Eligible patients with 2 baseline platelet counts <30×109/L received oral rilzabrutinib at starting doses of 200 mg QD, 400 mg QD, 300 mg BID, or 400 mg BID; intrapatient dose escalation was allowed to improve efficacy. Rilzabrutinib was given for 24 weeks; additional long-term therapy was administered at the 400 mg BID dose in responders only. Stable doses of concomitant ITP medication (corticosteroids and thrombopoietin-receptor agonists) were permitted for patients without adequate platelet response. The primary endpoints are safety and ≥2 consecutive platelet counts ≥50×109/L and increased ≥20×109/L from baseline without requiring rescue medication. All patients provided informed consent.
Results
The overall study population comprises 59 patients, of whom 44 had a starting dose of 400 mg BID; data cutoff was 09Nov2020. Overall, patients had a median age 50 years (range, 21-74), median duration of ITP 6.4 years (range, 0.4-52.5), and a median of 6.0 prior therapies (range, 1-53; 25% prior splenectomy). Baseline median platelet counts were 15-16×109/L (range, 2-33). Baseline demographics were similar for patients initiating 400 mg BID. A total of 23 patients (39%) overall and 17 (39%) initiating 400 mg BID achieved the primary efficacy endpoint (Table). Primary responses were observed irrespective of prior treatments or responses. Responders maintained platelet counts ≥50×109/L for a median of 75% of weeks, as well as increased platelet counts of ≥20×109/L above baseline levels for a median of 87% of weeks (Figure). Approximately half of all patients attained platelet counts of ≥30×109/L by day 8. Day 8 platelet count was associated with a high negative predictive value for achieving the primary endpoint (>80%) and may assist with identifying patients unlikely to reach the targeted primary platelet response. Related treatment-emergent adverse events (all grade 1/2) were observed in 29 of 59 (49%) patients overall; most common were 31% diarrhea and 25% nausea (all others were <10%). There were no related bleeding/thrombotic events, ECG abnormalities, hypertension, or liver enzyme elevations.

Conclusion
Rilzabrutinib was well-tolerated and showed rapid and durable responses, with half of the patients achieving platelet counts ≥30×109/L by day 8, potentially providing a tool for predicting early response to treatment, and a durable primary endpoint response. Taken together, these findings support further investigation of the magnitude and durability of rilzabrutinib clinical benefit.
Keyword(s): Immune thrombocytopenia (ITP), Platelet


