
Contributions
Abstract: S291
Type: Oral Presentation
Session title: Transfusion medicine
Background
CAD is a rare autoimmune hemolytic anemia mediated by classical complement pathway activation. Sutimlimab, a first-in-class humanized monoclonal anti-C1s G4 antibody, selectively inhibits this pathway, halts hemolysis, and improves hemoglobin (Hb) and quality of life (QOL) in CAD patients with recent transfusion history (pivotal single-arm CARDINAL study [NCT03347396]). CADENZA (NCT03347422) is a 26-week (Part A) randomized, double-blind, PBO-controlled Phase 3 study with open-label extension (Part B) to assess sutimlimab in CAD patients without recent transfusion history.
Aims
To report efficacy and safety results from CADENZA Part A.
Methods
Confirmed CAD-diagnosed patients with baseline Hb ≤10 g/dL, abnormally high bilirubin, transfusion independence, and ≥1 CAD symptom were enrolled after informed consent. Patients were randomized 1:1 to receive sutimlimab (<75 kg, 6.5 g; ≥75 kg, 7.5 g; N=22) or PBO (N=20) on Days 0 and 7, then biweekly infusions. The composite primary endpoint (sutimlimab vs PBO compared using the Cochran-Mantel-Haenszel method) was the proportion of patients with Hb increase ≥1.5 g/dL at the treatment assessment time point (TAT; mean of Weeks 23, 25, and 26), and avoidance of transfusion and study-prohibited CAD therapy (Weeks 5–26). Secondary endpoints (analyzed with mixed model for repeated measures and summary statistics) included TAT change from baseline in Hb, bilirubin, and Functional Assessment of Chronic Illness Therapy [FACIT]-Fatigue. Safety was evaluated.
Results
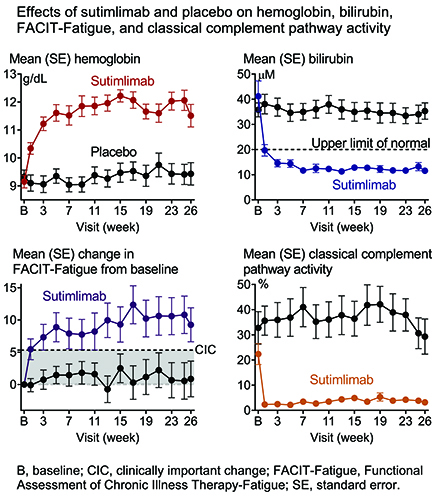
Baseline mean (standard deviation) g/dL Hb was 9.2 (1.1) for sutimlimab and 9.3 (1.0) for PBO. Composite primary endpoint criteria were met by 16 (73%) patients in sutimlimab and 3 (15%) in PBO (odds ratio, 15.9 [95% confidence interval, 2.9–88.0; P<0.001]). Sutimlimab, but not PBO, increased mean Hb and QOL (FACIT-Fatigue) and normalized mean bilirubin by Week 1, which were sustained to TAT (Figure). At TAT, the least squares mean (standard error) difference in Hb and FACIT-Fatigue between sutimlimab and PBO was 2.6 (0.4) g/dL (P<0.001) and 8.9 (2.5) points (P<0.001). Improvements coincided with near-complete classical pathway inhibition and C4 normalization. One (5%) patient in sutimlimab and 4 (20%) in PBO received transfusions from Weeks 5 to 26. There were 146 (62% of events) treatment-emergent adverse events (TEAE) in sutimlimab and 90 (38%) in PBO. Three (14%) sutimlimab and 1 (5%) PBO patients had ≥1 serious TEAE (TESAE). One TESAE of cerebral venous thrombosis was related to sutimlimab as assessed by the investigator. Serious infections were reported but none were meningococcal. No TESAE of hypersensitivity or anaphylaxis were reported. No TEAE was suggestive of the development/worsening of autoimmune disease or systemic lupus erythematosus. All PBO and 86% of sutimlimab patients completed Part A and entered Part B; 3 sutimlimab patients discontinued study due to TEAE (acrocyanosis and Raynaud's phenomenon [n=1], infusion-related reactions [n=1], increased blood immunoglobulin M [n=1]). Acrocyanosis and infusion-related reactions were assessed as related to sutimlimab by the investigator. Hypertension (23% vs 0%), Raynaud’s phenomenon (18% vs 0%), and acrocyanosis (14% vs 0%) were reported more often in the sutimlimab vs PBO group. No deaths were reported in either group.
Conclusion
Sutimlimab, but not PBO, rapidly halted hemolysis, markedly increased Hb, and improved QOL (fatigue). In addition to the single-arm CARDINAL study, the CADENZA PBO-controlled results support targeting C1s in CAD.
Keyword(s): Autoimmune hemolytic anemia (AIHA), Complement, Hemoglobin, Hemolysis
Abstract: S291
Type: Oral Presentation
Session title: Transfusion medicine
Background
CAD is a rare autoimmune hemolytic anemia mediated by classical complement pathway activation. Sutimlimab, a first-in-class humanized monoclonal anti-C1s G4 antibody, selectively inhibits this pathway, halts hemolysis, and improves hemoglobin (Hb) and quality of life (QOL) in CAD patients with recent transfusion history (pivotal single-arm CARDINAL study [NCT03347396]). CADENZA (NCT03347422) is a 26-week (Part A) randomized, double-blind, PBO-controlled Phase 3 study with open-label extension (Part B) to assess sutimlimab in CAD patients without recent transfusion history.
Aims
To report efficacy and safety results from CADENZA Part A.
Methods
Confirmed CAD-diagnosed patients with baseline Hb ≤10 g/dL, abnormally high bilirubin, transfusion independence, and ≥1 CAD symptom were enrolled after informed consent. Patients were randomized 1:1 to receive sutimlimab (<75 kg, 6.5 g; ≥75 kg, 7.5 g; N=22) or PBO (N=20) on Days 0 and 7, then biweekly infusions. The composite primary endpoint (sutimlimab vs PBO compared using the Cochran-Mantel-Haenszel method) was the proportion of patients with Hb increase ≥1.5 g/dL at the treatment assessment time point (TAT; mean of Weeks 23, 25, and 26), and avoidance of transfusion and study-prohibited CAD therapy (Weeks 5–26). Secondary endpoints (analyzed with mixed model for repeated measures and summary statistics) included TAT change from baseline in Hb, bilirubin, and Functional Assessment of Chronic Illness Therapy [FACIT]-Fatigue. Safety was evaluated.
Results
Baseline mean (standard deviation) g/dL Hb was 9.2 (1.1) for sutimlimab and 9.3 (1.0) for PBO. Composite primary endpoint criteria were met by 16 (73%) patients in sutimlimab and 3 (15%) in PBO (odds ratio, 15.9 [95% confidence interval, 2.9–88.0; P<0.001]). Sutimlimab, but not PBO, increased mean Hb and QOL (FACIT-Fatigue) and normalized mean bilirubin by Week 1, which were sustained to TAT (Figure). At TAT, the least squares mean (standard error) difference in Hb and FACIT-Fatigue between sutimlimab and PBO was 2.6 (0.4) g/dL (P<0.001) and 8.9 (2.5) points (P<0.001). Improvements coincided with near-complete classical pathway inhibition and C4 normalization. One (5%) patient in sutimlimab and 4 (20%) in PBO received transfusions from Weeks 5 to 26. There were 146 (62% of events) treatment-emergent adverse events (TEAE) in sutimlimab and 90 (38%) in PBO. Three (14%) sutimlimab and 1 (5%) PBO patients had ≥1 serious TEAE (TESAE). One TESAE of cerebral venous thrombosis was related to sutimlimab as assessed by the investigator. Serious infections were reported but none were meningococcal. No TESAE of hypersensitivity or anaphylaxis were reported. No TEAE was suggestive of the development/worsening of autoimmune disease or systemic lupus erythematosus. All PBO and 86% of sutimlimab patients completed Part A and entered Part B; 3 sutimlimab patients discontinued study due to TEAE (acrocyanosis and Raynaud's phenomenon [n=1], infusion-related reactions [n=1], increased blood immunoglobulin M [n=1]). Acrocyanosis and infusion-related reactions were assessed as related to sutimlimab by the investigator. Hypertension (23% vs 0%), Raynaud’s phenomenon (18% vs 0%), and acrocyanosis (14% vs 0%) were reported more often in the sutimlimab vs PBO group. No deaths were reported in either group.

Conclusion
Sutimlimab, but not PBO, rapidly halted hemolysis, markedly increased Hb, and improved QOL (fatigue). In addition to the single-arm CARDINAL study, the CADENZA PBO-controlled results support targeting C1s in CAD.
Keyword(s): Autoimmune hemolytic anemia (AIHA), Complement, Hemoglobin, Hemolysis


