
Contributions
Abstract: S286
Type: Oral Presentation
Session title: COVID-19 impact on hematology: How it started - How it’s going
Background
Patients (pts) with acute leukemia (AL) are at high risk of COVID-19 severe and lethal course. Intensive chemotherapy and immunosuppression in these patients are the possible causes of high mortality. Emerging data on risk factors and outcomes in this vulnerable patient population is aimed to support strategies of their medical care.
Aims
To evaluate treatment outcomes and risk factors in pts with AL and COVID-19 in a real-world setting.
Methods
CHRONOS19 [NCT04422470] is an ongoing nationwide observational cohort study of adult (≥18 y) pts with hematologic disease (both malignant and non-malignant) and lab-confirmed or suspected (based on clinical and/or CT findings) COVID-19. We performed a subgroup analysis of pts with AL and COVID-19. Primary objective was to evaluate treatment outcomes and identify risk factors associated with higher mortality in this group of pts. Primary endpoint was 30-day all-cause mortality. Long-term follow-up was performed at 90 and 180 days. Data from 14 centers was collected on a web-based platform and managed in a deidentified manner.
Results
As of data cutoff on January 27, 2021, 575 pts were included in the registry, 132 of them with AL were eligible for primary endpoint assessment n(%): AML – 86(65%), ALL – 37(28%), APL – 9(7%); M/F 55(42%)/77(58%), median age 45 [18-79] years, induction phase/R/R/treatment in remission 49(37%)/27(21%)/ 54(42%), agranulocytosis in 55(42%) pts, 68(53%) pts were transfusion dependent, comorbidities – in 58(45%) pts. Complications developed in 98(74%) pts: pneumonia (71%), sepsis (9%), CRS (8%), ARDS (6%). One-third of pts had severe COVID-19, 30% were admitted to ICU, 26% required mechanical ventilation. We performed comparative analysis of characteristics between a group of pts with AL and other hematologic diseases (lymphomas, chronic leukemia, multiple myeloma, and other malignant and non-malignant diseases): pts with AL had significantly more frequent agranulocytosis (42% vs 20%, p<0,001), transfusion dependence (53% vs 33%, p<0,001), and a higher rate of admission to ICU (30% vs 20%, p=0,036).
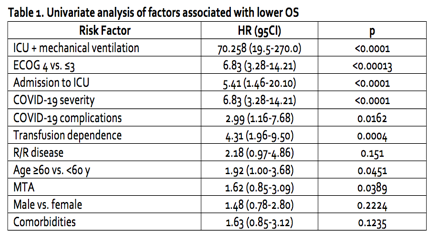
All-cause mortality at 30 days was 24% in pts with AL vs. 15% in pts with other hematologic diseases (p=0,03); 70% of deaths were due to COVID-19 complications in pts with AL. At 90 days, one additional death due to COVID-19 occurred. At 180 days, 5 more deaths due to leukemia progression were registered. Risk factors that were significantly associated with overall survival (OS) are listed in Table 1. In multivariate analysis, ICU + mechanical ventilation, HR, 70.258 (19.5-270.0) and age >60 years HR, 3.340 (1.08-10.9) were the most significant risk factors of death. Overall, AL were associated with a higher risk of death, HR, 2.40 (1.28-4.51), compared to less aggressive diseases (CML, CLL, MM, lymphomas, non-malignant), HR, 0.54 (0.37-0.80). COVID-19 affected treatment of AL in 65% of pts, 58% experienced treatment delay for a median of 4 [1-10] weeks. In 11 of 103 (10,7%) pts with AL early relapse/refractory disease was detected, but the association of relapses with affected treatment is unknown. COVID-19 re-infection was described in 2 patients.
Conclusion
Thirty-day all-cause mortality was significantly higher in SARS-CoV-2-infected pts with AL than in those with other hematologic diseases. Longer-term follow-up (180 days) for AL outcomes and OS will be presented.
Keyword(s): COVID-19, Leukemia, Outcome measurement
Abstract: S286
Type: Oral Presentation
Session title: COVID-19 impact on hematology: How it started - How it’s going
Background
Patients (pts) with acute leukemia (AL) are at high risk of COVID-19 severe and lethal course. Intensive chemotherapy and immunosuppression in these patients are the possible causes of high mortality. Emerging data on risk factors and outcomes in this vulnerable patient population is aimed to support strategies of their medical care.
Aims
To evaluate treatment outcomes and risk factors in pts with AL and COVID-19 in a real-world setting.
Methods
CHRONOS19 [NCT04422470] is an ongoing nationwide observational cohort study of adult (≥18 y) pts with hematologic disease (both malignant and non-malignant) and lab-confirmed or suspected (based on clinical and/or CT findings) COVID-19. We performed a subgroup analysis of pts with AL and COVID-19. Primary objective was to evaluate treatment outcomes and identify risk factors associated with higher mortality in this group of pts. Primary endpoint was 30-day all-cause mortality. Long-term follow-up was performed at 90 and 180 days. Data from 14 centers was collected on a web-based platform and managed in a deidentified manner.
Results
As of data cutoff on January 27, 2021, 575 pts were included in the registry, 132 of them with AL were eligible for primary endpoint assessment n(%): AML – 86(65%), ALL – 37(28%), APL – 9(7%); M/F 55(42%)/77(58%), median age 45 [18-79] years, induction phase/R/R/treatment in remission 49(37%)/27(21%)/ 54(42%), agranulocytosis in 55(42%) pts, 68(53%) pts were transfusion dependent, comorbidities – in 58(45%) pts. Complications developed in 98(74%) pts: pneumonia (71%), sepsis (9%), CRS (8%), ARDS (6%). One-third of pts had severe COVID-19, 30% were admitted to ICU, 26% required mechanical ventilation. We performed comparative analysis of characteristics between a group of pts with AL and other hematologic diseases (lymphomas, chronic leukemia, multiple myeloma, and other malignant and non-malignant diseases): pts with AL had significantly more frequent agranulocytosis (42% vs 20%, p<0,001), transfusion dependence (53% vs 33%, p<0,001), and a higher rate of admission to ICU (30% vs 20%, p=0,036).
All-cause mortality at 30 days was 24% in pts with AL vs. 15% in pts with other hematologic diseases (p=0,03); 70% of deaths were due to COVID-19 complications in pts with AL. At 90 days, one additional death due to COVID-19 occurred. At 180 days, 5 more deaths due to leukemia progression were registered. Risk factors that were significantly associated with overall survival (OS) are listed in Table 1. In multivariate analysis, ICU + mechanical ventilation, HR, 70.258 (19.5-270.0) and age >60 years HR, 3.340 (1.08-10.9) were the most significant risk factors of death. Overall, AL were associated with a higher risk of death, HR, 2.40 (1.28-4.51), compared to less aggressive diseases (CML, CLL, MM, lymphomas, non-malignant), HR, 0.54 (0.37-0.80). COVID-19 affected treatment of AL in 65% of pts, 58% experienced treatment delay for a median of 4 [1-10] weeks. In 11 of 103 (10,7%) pts with AL early relapse/refractory disease was detected, but the association of relapses with affected treatment is unknown. COVID-19 re-infection was described in 2 patients.

Conclusion
Thirty-day all-cause mortality was significantly higher in SARS-CoV-2-infected pts with AL than in those with other hematologic diseases. Longer-term follow-up (180 days) for AL outcomes and OS will be presented.
Keyword(s): COVID-19, Leukemia, Outcome measurement


