
Contributions
This abstract is embargoed until Saturday, June 12, 09:00 CEST
Abstract: S285
Type: Oral Presentation
Session title: COVID-19 impact on hematology: How it started - How it’s going
Background
The Pfizer/BioNTech BNT162b2 vaccine, employing mRNA technology, has been recently approved by both the FDA and EMA for the prevention of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, demonstrating a 94.6% protection rate in a phase 3 study. While this vaccine is recommended by the FDA, EBMT and ASH-ASTCT for immunosuppressed patients, data regarding protection efficacy and safety in patients undergoing immunologic cell therapy are scarce.
Aims
We aimed to evaluate efficacy and toxicity of the BNT162b2 vaccine in patients that underwent hematopoietic cell transplantation and CAR-T therapy.
Methods
All patients under active treatment at the long-term follow-up HCT clinic (n=124) at the Tel Aviv Sourasky Medical Center, were evaluated for immunologic recovery (CD19+, CD4+, and CD8+ cell blood levels) pre-vaccination and were recommended to receive the commercial vaccination based on the EBMT recommendations. Patients were prospectively followed for vaccination-safety profile (laboratory tests, GVHD monitoring, and symptom-based questionnaire). We evaluated the humoral immune response to vaccine, 7-14 days after the second vaccine dose, by in vitro quantitative determination of anti-SARS-CoV-2S antibodies using Elecsys® assay and cellular immune response by ELISpot, estimating IL-2 and IFN-gamma secretion in response to a pool of lyophilized SARS-COV-2 S and M peptides (PepTivator; Miltenyi). The trial was approved by the local Ethics Committee and was registered by the clinical trials network (NCT04724642).
Results
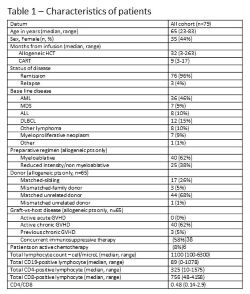
From 23-Dec-2020 all sequential patients (allogeneic, n=101 and CAR-T, n=23) were assessed for eligibility based on the EBMT recommendations (Version 5.0, Feb 21, 2021). Of those, 100 patients were eligible and 79 patients (allogeneic, n=65 and CAR-T, n=14) were vaccinated per-protocol. Characteristics of patients are depicted in Table 1. Overall, the 2 vaccine doses were well tolerated. Adverse events were reported in 39% of allogeneic HCT recipients (4.6% grade ≥3) and 32% of CART recipients (7% grade ≥3). All events resolved within few days, with the exception of 1 secondary graft rejection which is still under investigation. Among the CAR-T group, 5 patients (36%) had humoral antibody response. Patients with CD19+ lymphocytes >0 had a higher likelihood to develop antibodies compared to those with B cell aplasia (67% vs. 12.5%, p=.036). Among the allogeneic HCT group - 47 patients (81%) had a humoral antibody response. Incidence of positive serology was lower in patients with concomitant high intensity immunosuppressive therapy (IST) compared to those with low intensity IST (69% vs. 94%, p=.016). Linear regressions identified that male sex (beta=-.380, p=.012) and high intensity IST (beta=-.497, p=.014) were associated with lower antibody titer, while age, months from HCT, intensity of conditioning, low CD19 cell count, and active GVHD did not predict response. Analysis of peptide induced cytokine release by ELISpot is ongoing and will be presented at the EHA meeting.
Conclusion
Humoral response to the BNT162b2 mRNA COVID-19 vaccine in CAR-T patients with B cell aplasia is significantly impaired, while overall response in patients after allogeneic HCT is encouraging. Patients on concomitant high intensity IST had impaired humoral response to BNT162b2. Longer follow-up is mandatory to test persistence of antibodies, and general preventive practices should be continued until more data are available.
Keyword(s): Allogeneic hematopoietic stem cell transplant, CAR-T, COVID-19, Vaccination
This abstract is embargoed until Saturday, June 12, 09:00 CEST
Abstract: S285
Type: Oral Presentation
Session title: COVID-19 impact on hematology: How it started - How it’s going
Background
The Pfizer/BioNTech BNT162b2 vaccine, employing mRNA technology, has been recently approved by both the FDA and EMA for the prevention of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, demonstrating a 94.6% protection rate in a phase 3 study. While this vaccine is recommended by the FDA, EBMT and ASH-ASTCT for immunosuppressed patients, data regarding protection efficacy and safety in patients undergoing immunologic cell therapy are scarce.
Aims
We aimed to evaluate efficacy and toxicity of the BNT162b2 vaccine in patients that underwent hematopoietic cell transplantation and CAR-T therapy.
Methods
All patients under active treatment at the long-term follow-up HCT clinic (n=124) at the Tel Aviv Sourasky Medical Center, were evaluated for immunologic recovery (CD19+, CD4+, and CD8+ cell blood levels) pre-vaccination and were recommended to receive the commercial vaccination based on the EBMT recommendations. Patients were prospectively followed for vaccination-safety profile (laboratory tests, GVHD monitoring, and symptom-based questionnaire). We evaluated the humoral immune response to vaccine, 7-14 days after the second vaccine dose, by in vitro quantitative determination of anti-SARS-CoV-2S antibodies using Elecsys® assay and cellular immune response by ELISpot, estimating IL-2 and IFN-gamma secretion in response to a pool of lyophilized SARS-COV-2 S and M peptides (PepTivator; Miltenyi). The trial was approved by the local Ethics Committee and was registered by the clinical trials network (NCT04724642).
Results
From 23-Dec-2020 all sequential patients (allogeneic, n=101 and CAR-T, n=23) were assessed for eligibility based on the EBMT recommendations (Version 5.0, Feb 21, 2021). Of those, 100 patients were eligible and 79 patients (allogeneic, n=65 and CAR-T, n=14) were vaccinated per-protocol. Characteristics of patients are depicted in Table 1. Overall, the 2 vaccine doses were well tolerated. Adverse events were reported in 39% of allogeneic HCT recipients (4.6% grade ≥3) and 32% of CART recipients (7% grade ≥3). All events resolved within few days, with the exception of 1 secondary graft rejection which is still under investigation. Among the CAR-T group, 5 patients (36%) had humoral antibody response. Patients with CD19+ lymphocytes >0 had a higher likelihood to develop antibodies compared to those with B cell aplasia (67% vs. 12.5%, p=.036). Among the allogeneic HCT group - 47 patients (81%) had a humoral antibody response. Incidence of positive serology was lower in patients with concomitant high intensity immunosuppressive therapy (IST) compared to those with low intensity IST (69% vs. 94%, p=.016). Linear regressions identified that male sex (beta=-.380, p=.012) and high intensity IST (beta=-.497, p=.014) were associated with lower antibody titer, while age, months from HCT, intensity of conditioning, low CD19 cell count, and active GVHD did not predict response. Analysis of peptide induced cytokine release by ELISpot is ongoing and will be presented at the EHA meeting.

Conclusion
Humoral response to the BNT162b2 mRNA COVID-19 vaccine in CAR-T patients with B cell aplasia is significantly impaired, while overall response in patients after allogeneic HCT is encouraging. Patients on concomitant high intensity IST had impaired humoral response to BNT162b2. Longer follow-up is mandatory to test persistence of antibodies, and general preventive practices should be continued until more data are available.
Keyword(s): Allogeneic hematopoietic stem cell transplant, CAR-T, COVID-19, Vaccination


