
Contributions
Abstract: S282
Type: Oral Presentation
Session title: Fungal, bacterial and viral infections in hematology
Background
Prophylactic immunoglobulin (Ig) replacement and prophylactic oral antibiotics (PA) are used to prevent infections in patients with haematological malignancies and acquired hypogammaglobulinemia. Ig has been shown to reduce infection risk, but is costly and in limited supply. PA have been shown to reduce infection risk in other patient populations and are less expensive, but have side-effects and can increase antimicrobial resistance rates. Guidelines and clinical practice vary internationally, with some recommending a trial of PA prior to commencing Ig replacement, and others omitting PA. The efficacy, safety and cost-effectiveness of Ig and PA have not been directly compared in a randomised controlled trial (RCT) in patients with acquired hypogammaglobulinemia secondary to haematological malignancies.
Aims
To determine the feasibility of delivering PA as an alternative to Ig replacement in patients with haematological malignancies and acquired hypogammaglobulinemia.
Methods
Phase II, multicentre, feasibility RCT (ACTRN12616001723471). Eligible patients had acquired hypogammaglobulinemia due to a haematological malignancy, a history of recurrent or severe bacterial infections or an IgG level <4g/L (excluding paraprotein), and had a life expectancy more than 12 months. Exclusion criteria included prior allogeneic haematopoietic stem cell transplant and prior Ig replacement in the preceding 3 months. Patients were randomised to receive Ig (0.4g/kg IV every 4 weeks, modified to achieve an IgG trough level ≥ lower limit of reference range; or 0.1g/kg/week SC, modified to achieve an IgG trough level of ≥ lower limit of reference rage) or daily oral prophylactic antibiotics (trimethoprim-sulfamethoxazole 160mg/800mg, with 100mg doxycycline as an alternative for hypersensitivity) for 12 months at a 1:2 ratio. Randomisation was stratified by site. Patients allocated to PA were allowed to cross over to Ig if they experienced a CTCAE ≥ Grade 3 infection. Treatment allocation was not blinded but infectious outcomes were adjudicated by an independent, blinded, outcome adjudication committee. The primary outcome was proportion of patients alive and remaining on assigned treatment arm 12 months following randomisation. Secondary outcomes included time to first major infection (defined as CTCAE ≥ Grade 3 infection).
Results
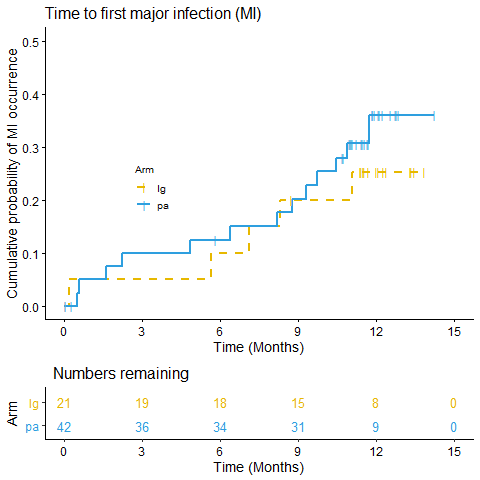
63 patients were randomised, 60 received treatment and had primary outcome data available. The median age was 69 years (IQR 66-75), 34 (54%) were female, 12 (19%) had myeloma, 29 (46%) chronic lymphocytic leukaemia, 20 (32%) had non-Hodgkin lymphoma and 2 (3%) other haematological malignancy. For the primary outcome, 76% (95% CI 53-92) in the Ig arm and 71% (95% CI 55-84) in the PA arm were alive and on assigned treatment 12 months following randomisation (p=0.77). The time to first major infection was similar between the two groups (see figure 1), with the lower quartile 11.0 months in Ig arm vs 9.7 months in PA arm, (log rank test p=0.54). 74% of patients in the Ig arm and 64% in the antibiotic arm were free of major infection during the 12 months.
Conclusion
In this phase II trial, a similar proportion of patients allocated to Ig and to PA remained alive and on their assigned treatment arm at 12 months. There was no significant difference in time to first major infection between the two treatment arms. These findings support the feasibility of proceeding with a phase III trial to compare efficacy, safety and cost-effectiveness of Ig replacement to PA in patients with acquired hypogammaglobulinemia.
Keyword(s): Clinical trial, Infection, Intravenous gamma-globulin, Prophylaxis
Abstract: S282
Type: Oral Presentation
Session title: Fungal, bacterial and viral infections in hematology
Background
Prophylactic immunoglobulin (Ig) replacement and prophylactic oral antibiotics (PA) are used to prevent infections in patients with haematological malignancies and acquired hypogammaglobulinemia. Ig has been shown to reduce infection risk, but is costly and in limited supply. PA have been shown to reduce infection risk in other patient populations and are less expensive, but have side-effects and can increase antimicrobial resistance rates. Guidelines and clinical practice vary internationally, with some recommending a trial of PA prior to commencing Ig replacement, and others omitting PA. The efficacy, safety and cost-effectiveness of Ig and PA have not been directly compared in a randomised controlled trial (RCT) in patients with acquired hypogammaglobulinemia secondary to haematological malignancies.
Aims
To determine the feasibility of delivering PA as an alternative to Ig replacement in patients with haematological malignancies and acquired hypogammaglobulinemia.
Methods
Phase II, multicentre, feasibility RCT (ACTRN12616001723471). Eligible patients had acquired hypogammaglobulinemia due to a haematological malignancy, a history of recurrent or severe bacterial infections or an IgG level <4g/L (excluding paraprotein), and had a life expectancy more than 12 months. Exclusion criteria included prior allogeneic haematopoietic stem cell transplant and prior Ig replacement in the preceding 3 months. Patients were randomised to receive Ig (0.4g/kg IV every 4 weeks, modified to achieve an IgG trough level ≥ lower limit of reference range; or 0.1g/kg/week SC, modified to achieve an IgG trough level of ≥ lower limit of reference rage) or daily oral prophylactic antibiotics (trimethoprim-sulfamethoxazole 160mg/800mg, with 100mg doxycycline as an alternative for hypersensitivity) for 12 months at a 1:2 ratio. Randomisation was stratified by site. Patients allocated to PA were allowed to cross over to Ig if they experienced a CTCAE ≥ Grade 3 infection. Treatment allocation was not blinded but infectious outcomes were adjudicated by an independent, blinded, outcome adjudication committee. The primary outcome was proportion of patients alive and remaining on assigned treatment arm 12 months following randomisation. Secondary outcomes included time to first major infection (defined as CTCAE ≥ Grade 3 infection).
Results
63 patients were randomised, 60 received treatment and had primary outcome data available. The median age was 69 years (IQR 66-75), 34 (54%) were female, 12 (19%) had myeloma, 29 (46%) chronic lymphocytic leukaemia, 20 (32%) had non-Hodgkin lymphoma and 2 (3%) other haematological malignancy. For the primary outcome, 76% (95% CI 53-92) in the Ig arm and 71% (95% CI 55-84) in the PA arm were alive and on assigned treatment 12 months following randomisation (p=0.77). The time to first major infection was similar between the two groups (see figure 1), with the lower quartile 11.0 months in Ig arm vs 9.7 months in PA arm, (log rank test p=0.54). 74% of patients in the Ig arm and 64% in the antibiotic arm were free of major infection during the 12 months.

Conclusion
In this phase II trial, a similar proportion of patients allocated to Ig and to PA remained alive and on their assigned treatment arm at 12 months. There was no significant difference in time to first major infection between the two treatment arms. These findings support the feasibility of proceeding with a phase III trial to compare efficacy, safety and cost-effectiveness of Ig replacement to PA in patients with acquired hypogammaglobulinemia.
Keyword(s): Clinical trial, Infection, Intravenous gamma-globulin, Prophylaxis


