
Contributions
Abstract: S280
Type: Oral Presentation
Session title: Fungal, bacterial and viral infections in hematology
Background
Efficacy of vaccines against SARS-CoV-2 virus is of great importance in order to mitigate COVID-19 pandemic. The BNT162b2 mRNA vaccine is the first approved by both FDA and EMA vaccine with confirmed clinical activity in phase 3 trials. However, the immune responses against SARS-CoV-2 after BNT162b2 vaccination have not been fully elucidated in several population subsets including octogenarians and patients with malignancies or autoimmune disorders.
Aims
The aim of this prospective study (study no: NCT04743388) is to evaluate the development of neutralizing (NAbs) and anti-spike RBD IgG antibodies against SARS-CoV-2, as well as the levels of cytokines and the induced B- and T-cells subpopulations following vaccination with BNT162b2 vaccine. Participating groups include healthy sanitary workers, elderly individuals (above the age of 70) and patients with hematological malignancies or solid tumors under different types of treatment. Herein we present preliminary results of the BNT162b2 vaccine effects in the development of anti-SARS-CoV-2 antibodies in healthy sanitary workers and octogenarians.
Methods
Healthy sanitary (i.e., physicians and nurses) workers of Alexandra hospital, Athens, Greece and volunteered octogenarians were included in this study. The inclusion criteria included (a) age higher than 18 years and (b) the absence of any known auto-immune or malignant disease. NAbs against SARS-CoV-2 and anti-Spike-RBD IgG antibodies were measured using FDA approved methods, i.e., the cPass™ SARS-CoV-2 Neutralization Antibody Detection Kit (GenScript, Piscataway, NJ, USA) and the Elecsys Anti-SARS-CoV-2 S assay (Roche Diagnostics GmbH, Mannheim, Germany), respectively. Time-points for blood collection were day 1 (D1) (first BNT162b2 dose), D8, D22 (second dose), D36 and D50 for healthy sanitary workers and D1, D22 and D50 for octogenarians. The study includes also a follow-up of the antibodies levels every 3 months till month 18 post the delivery of the second dose (D22) of the vaccine.
Results
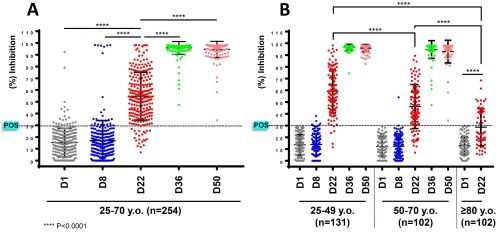
The study population included 254 healthy sanitary workers (92 males/162 females; median age: 49 years, range: 25-70 years) (group 1) and 112 octogenarians (51 males/61 females; median age: 85 years, range: 80-95 years) (group 2). Twenty-one donors from group one and ten from group two had NAbs inhibition titers >30% (threshold for positivity) at D1; these titers significantly expanded at D8 indicating the presence of sustained rapid anti-SARS-CoV-2 antibody production in COVID-19 healers (Figure, A). NAbs titers (in negative at D1 donors) from group one, were found to increase significantly already at D22 (day of second vaccination), plateaued two weeks (D36) after the second dose of the vaccine (NAbs inhibition ≥90%) and remained at high levels at D50 (NAbs inhibition ≥90%) (Figure, A). Notably, the NAbs titers (in negative at D1 donors) had lower increases in ages 50-70 vs. 25-49 at D22; this age-related effect of the vaccine efficacy was confirmed in octogenarians who at D22 developed significantly lower NAbs titers vs. all other age groups (Figure, B). Similar kinetics were found in donors of groups one and two for the anti-spike RBD IgG antibodies.
Conclusion
We conclude that the BNT162b2 mRNA vaccine is exceedingly effective in producing high anti-SARS-CoV-2 antibody (NAbs and anti-spike RBD IgGs) titers in healthy donors. Given however that this readout is seemingly (up to the delivery of the second dose of the vaccine) age-dependent, we suggest that the second timely vaccination is needed, especially in the elderly population.
Keyword(s): Antibody, COVID-19
Abstract: S280
Type: Oral Presentation
Session title: Fungal, bacterial and viral infections in hematology
Background
Efficacy of vaccines against SARS-CoV-2 virus is of great importance in order to mitigate COVID-19 pandemic. The BNT162b2 mRNA vaccine is the first approved by both FDA and EMA vaccine with confirmed clinical activity in phase 3 trials. However, the immune responses against SARS-CoV-2 after BNT162b2 vaccination have not been fully elucidated in several population subsets including octogenarians and patients with malignancies or autoimmune disorders.
Aims
The aim of this prospective study (study no: NCT04743388) is to evaluate the development of neutralizing (NAbs) and anti-spike RBD IgG antibodies against SARS-CoV-2, as well as the levels of cytokines and the induced B- and T-cells subpopulations following vaccination with BNT162b2 vaccine. Participating groups include healthy sanitary workers, elderly individuals (above the age of 70) and patients with hematological malignancies or solid tumors under different types of treatment. Herein we present preliminary results of the BNT162b2 vaccine effects in the development of anti-SARS-CoV-2 antibodies in healthy sanitary workers and octogenarians.
Methods
Healthy sanitary (i.e., physicians and nurses) workers of Alexandra hospital, Athens, Greece and volunteered octogenarians were included in this study. The inclusion criteria included (a) age higher than 18 years and (b) the absence of any known auto-immune or malignant disease. NAbs against SARS-CoV-2 and anti-Spike-RBD IgG antibodies were measured using FDA approved methods, i.e., the cPass™ SARS-CoV-2 Neutralization Antibody Detection Kit (GenScript, Piscataway, NJ, USA) and the Elecsys Anti-SARS-CoV-2 S assay (Roche Diagnostics GmbH, Mannheim, Germany), respectively. Time-points for blood collection were day 1 (D1) (first BNT162b2 dose), D8, D22 (second dose), D36 and D50 for healthy sanitary workers and D1, D22 and D50 for octogenarians. The study includes also a follow-up of the antibodies levels every 3 months till month 18 post the delivery of the second dose (D22) of the vaccine.
Results
The study population included 254 healthy sanitary workers (92 males/162 females; median age: 49 years, range: 25-70 years) (group 1) and 112 octogenarians (51 males/61 females; median age: 85 years, range: 80-95 years) (group 2). Twenty-one donors from group one and ten from group two had NAbs inhibition titers >30% (threshold for positivity) at D1; these titers significantly expanded at D8 indicating the presence of sustained rapid anti-SARS-CoV-2 antibody production in COVID-19 healers (Figure, A). NAbs titers (in negative at D1 donors) from group one, were found to increase significantly already at D22 (day of second vaccination), plateaued two weeks (D36) after the second dose of the vaccine (NAbs inhibition ≥90%) and remained at high levels at D50 (NAbs inhibition ≥90%) (Figure, A). Notably, the NAbs titers (in negative at D1 donors) had lower increases in ages 50-70 vs. 25-49 at D22; this age-related effect of the vaccine efficacy was confirmed in octogenarians who at D22 developed significantly lower NAbs titers vs. all other age groups (Figure, B). Similar kinetics were found in donors of groups one and two for the anti-spike RBD IgG antibodies.

Conclusion
We conclude that the BNT162b2 mRNA vaccine is exceedingly effective in producing high anti-SARS-CoV-2 antibody (NAbs and anti-spike RBD IgGs) titers in healthy donors. Given however that this readout is seemingly (up to the delivery of the second dose of the vaccine) age-dependent, we suggest that the second timely vaccination is needed, especially in the elderly population.
Keyword(s): Antibody, COVID-19


