
Contributions
Abstract: S278
Type: Oral Presentation
Session title: Focus on iron metabolism
Background
Acute myeloid leukemia (AML) is a heterogeneous disease with poor prognosis and limited treatment strategies. Determining the role of cell-extrinsic regulators of leukemic cells is vital to gain clinical insights into the biology of AML. Iron is a key extrinsic regulator of cancer but its systemic regulation remains poorly explored in AML.
Aims
We aimed at studying iron metabolism in AML and the mechanisms involved in its regulation.
Methods
We analyzed AML patients at diagnosis and explored mechanisms involved in the regulation of iron metabolism using the syngeneic MLL-AF9-induced AML mouse model and mouse mutants.
Results
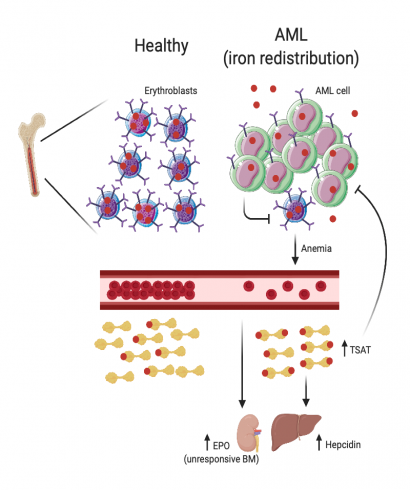
We analyzed serum iron parameters of 84 AML patients at diagnosis and observed a unique profile not associated with inflammation or red blood cell (RBC) transfusions: high ferritin, low transferrin, normal to high serum iron and elevated TSAT (median 51.5%). We also observed that AML patients had very high circulating hepcidin levels (median 152.0 vs. 14.3 ng/ml; p< 0.001), albeit a modest increase in inflammatory C-reactive protein (CRP) and interleukin 6 (IL-6). To explore the mechanism underlying the changes of iron metabolism in AML, we used the syngeneic MLL-AF9 AML mouse model. We observed that only infiltrated mice treated with chemotherapy had increased TSAT. Interestingly, AML-burdened mice had hyperleukocytosis with lymphopenia and thrombocytopenia but normal RBC and reticulocyte counts. A key difference between adult mice and humans is the significant contribution of the spleen to mouse erythropoiesis. We observed that bone marrow (BM) erythroblasts were depleted in human and mouse AML but splenic erythropoiesis was enhanced in leukemic mice. We hypothesized that the loss of erythroblasts in AML could also drive iron redistribution and TSAT increase in mice, if not compensated by an increased splenic erythropoiesis. As predicted, splenectomized mice with AML had anemia, reticulocytopenia, increased TSAT and detectable non-transferrin bound iron (NTBI), demonstrating that loss of BM erythroblasts in AML causes iron redistribution. Through direct quantification, protein and transcriptomic analysis, we demonstrated that iron is partially redistributed into AML cells. This led us to hypothesize that iron redistribution may be clinically relevant in AML. Survival analysis revealed that AML patients with higher TSAT at diagnosis had significantly better overall survival (OS), even after multivariate analysis. Experiments using different mouse models (Hfe-KO, iron-dextran) confirmed that increased TSAT is associated with better OS in AML.
Conclusion
Our study shows that AML patients have a unique iron profile independent of RBC transfusions characterized by increased TSAT due to loss of BM erythroblasts. We propose that TSAT at diagnosis may be a relevant independent prognostic factor in AML.
Keyword(s): Acute myeloid leukemia, Iron metabolism
Abstract: S278
Type: Oral Presentation
Session title: Focus on iron metabolism
Background
Acute myeloid leukemia (AML) is a heterogeneous disease with poor prognosis and limited treatment strategies. Determining the role of cell-extrinsic regulators of leukemic cells is vital to gain clinical insights into the biology of AML. Iron is a key extrinsic regulator of cancer but its systemic regulation remains poorly explored in AML.
Aims
We aimed at studying iron metabolism in AML and the mechanisms involved in its regulation.
Methods
We analyzed AML patients at diagnosis and explored mechanisms involved in the regulation of iron metabolism using the syngeneic MLL-AF9-induced AML mouse model and mouse mutants.
Results
We analyzed serum iron parameters of 84 AML patients at diagnosis and observed a unique profile not associated with inflammation or red blood cell (RBC) transfusions: high ferritin, low transferrin, normal to high serum iron and elevated TSAT (median 51.5%). We also observed that AML patients had very high circulating hepcidin levels (median 152.0 vs. 14.3 ng/ml; p< 0.001), albeit a modest increase in inflammatory C-reactive protein (CRP) and interleukin 6 (IL-6). To explore the mechanism underlying the changes of iron metabolism in AML, we used the syngeneic MLL-AF9 AML mouse model. We observed that only infiltrated mice treated with chemotherapy had increased TSAT. Interestingly, AML-burdened mice had hyperleukocytosis with lymphopenia and thrombocytopenia but normal RBC and reticulocyte counts. A key difference between adult mice and humans is the significant contribution of the spleen to mouse erythropoiesis. We observed that bone marrow (BM) erythroblasts were depleted in human and mouse AML but splenic erythropoiesis was enhanced in leukemic mice. We hypothesized that the loss of erythroblasts in AML could also drive iron redistribution and TSAT increase in mice, if not compensated by an increased splenic erythropoiesis. As predicted, splenectomized mice with AML had anemia, reticulocytopenia, increased TSAT and detectable non-transferrin bound iron (NTBI), demonstrating that loss of BM erythroblasts in AML causes iron redistribution. Through direct quantification, protein and transcriptomic analysis, we demonstrated that iron is partially redistributed into AML cells. This led us to hypothesize that iron redistribution may be clinically relevant in AML. Survival analysis revealed that AML patients with higher TSAT at diagnosis had significantly better overall survival (OS), even after multivariate analysis. Experiments using different mouse models (Hfe-KO, iron-dextran) confirmed that increased TSAT is associated with better OS in AML.

Conclusion
Our study shows that AML patients have a unique iron profile independent of RBC transfusions characterized by increased TSAT due to loss of BM erythroblasts. We propose that TSAT at diagnosis may be a relevant independent prognostic factor in AML.
Keyword(s): Acute myeloid leukemia, Iron metabolism


