
Contributions
Abstract: S274
Type: Oral Presentation
Session title: Changing the scene on congenital anemias
Background
Hereditary spherocytosis (HS) is one of the most common forms of hereditary hemolytic anemia in people of northern European descent (1:2000-3000). It is characterized by altered red blood cell (RBC) membrane integrity due to disruption of the (vertical) association between the cytoskeleton and the lipid bilayer. The disorder is highly heterogeneous, both clinically and molecularly, and genotype-phenotype correlations are largely unclear. The mechanistic basis of decreased red blood cell (RBC) deformability in HS has been well established but little is known about the metabolic consequences of reduced membrane-cytoskeleton integrity in HS. This question is especially relevant since RBC membrane proteins are implicated in cellular metabolism. In this study we investigated the metabolic impact of membrane defects using untargeted metabolomics in dried blood spots (DBS) and established a metabolic fingerprint for HS.
Aims
Investigating broad metabolic changes in HS by defining a metabolic signature for HS could 1. contribute to increased understanding of clinical heterogeneity, and 2. provide new leads for studying pathophysiological mechanisms in HS.
Methods
Untargeted metabolomics was applied on DBS samples obtained from 35 HS patients and 50 healthy controls by direct infusion high resolution mass spectrometry using a previously established protocol. (1,2) Statistical analyses were performed using MetaboAnalyst and Graphpad Prism (Version 8.3.0;538). Red blood cell deformability was evaluated by osmotic ektacytometry on the Laser Optical Rotational Red Cell Analyzer (Lorrca, RR Mechatronics, Zwaag, Netherlands)
Results
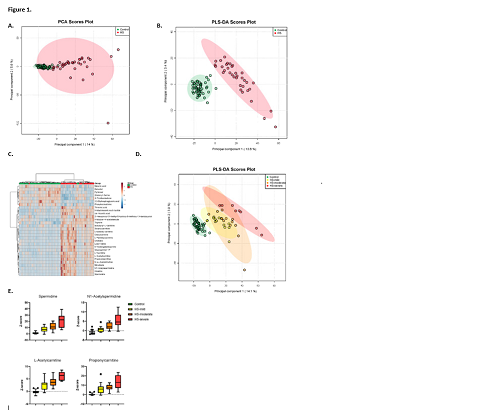
In total, 1770 unique metabolite features were identified in the respective DBS samples from patients and controls. Distinct metabolic profiles between groups were identified by principal component analysis and partial least square discriminant analysis (Figure 1A-B). Distinctive increased metabolites in the HS-fingerprint include polyamines and (acyl)carnitines. Vitamin B6 intermediates, 2,3-diphosphoglyceric acid and glyceraldehyde 3-phosphate were significantly decreased in HS (p<0.0001) (Figure 1C). Interestingly, when exploring phenotypic heterogeneity, subgroups of mildly, moderately and severely affected patients could be identified, revealing discrete differences in metabolic profiles for especially the mildly and severely affected patients (Figure 1D). Furthermore, the most discriminating metabolites in these metabolic profiles showed a gradient that relates with clinical disease severity (Figure 1E), and they correlated inversely and statistically significant with red cell characteristics. In addition, a trend to significance was observed for these metabolites and red blood cell deformability as measured by osmotic gradient ektacytometry.
Conclusion
In this study we identified a metabolic fingerprint for patients with HS using untargeted metabolomics in dried blood spots. The metabolic disturbances include altered levels of 2,3-DPG and polyamines, that correlate with red cell characteristics and clinical severity.
Hence, our data show that untargeted metabolomics in DBS is instrumental in investigating phenotypic heterogeneity and it provides promising leads for studying pathophysiological mechanisms in HS.
Keyword(s): Hemolytic anemia, Hereditary spherocytosis, Red blood cell
Abstract: S274
Type: Oral Presentation
Session title: Changing the scene on congenital anemias
Background
Hereditary spherocytosis (HS) is one of the most common forms of hereditary hemolytic anemia in people of northern European descent (1:2000-3000). It is characterized by altered red blood cell (RBC) membrane integrity due to disruption of the (vertical) association between the cytoskeleton and the lipid bilayer. The disorder is highly heterogeneous, both clinically and molecularly, and genotype-phenotype correlations are largely unclear. The mechanistic basis of decreased red blood cell (RBC) deformability in HS has been well established but little is known about the metabolic consequences of reduced membrane-cytoskeleton integrity in HS. This question is especially relevant since RBC membrane proteins are implicated in cellular metabolism. In this study we investigated the metabolic impact of membrane defects using untargeted metabolomics in dried blood spots (DBS) and established a metabolic fingerprint for HS.
Aims
Investigating broad metabolic changes in HS by defining a metabolic signature for HS could 1. contribute to increased understanding of clinical heterogeneity, and 2. provide new leads for studying pathophysiological mechanisms in HS.
Methods
Untargeted metabolomics was applied on DBS samples obtained from 35 HS patients and 50 healthy controls by direct infusion high resolution mass spectrometry using a previously established protocol. (1,2) Statistical analyses were performed using MetaboAnalyst and Graphpad Prism (Version 8.3.0;538). Red blood cell deformability was evaluated by osmotic ektacytometry on the Laser Optical Rotational Red Cell Analyzer (Lorrca, RR Mechatronics, Zwaag, Netherlands)
Results
In total, 1770 unique metabolite features were identified in the respective DBS samples from patients and controls. Distinct metabolic profiles between groups were identified by principal component analysis and partial least square discriminant analysis (Figure 1A-B). Distinctive increased metabolites in the HS-fingerprint include polyamines and (acyl)carnitines. Vitamin B6 intermediates, 2,3-diphosphoglyceric acid and glyceraldehyde 3-phosphate were significantly decreased in HS (p<0.0001) (Figure 1C). Interestingly, when exploring phenotypic heterogeneity, subgroups of mildly, moderately and severely affected patients could be identified, revealing discrete differences in metabolic profiles for especially the mildly and severely affected patients (Figure 1D). Furthermore, the most discriminating metabolites in these metabolic profiles showed a gradient that relates with clinical disease severity (Figure 1E), and they correlated inversely and statistically significant with red cell characteristics. In addition, a trend to significance was observed for these metabolites and red blood cell deformability as measured by osmotic gradient ektacytometry.

Conclusion
In this study we identified a metabolic fingerprint for patients with HS using untargeted metabolomics in dried blood spots. The metabolic disturbances include altered levels of 2,3-DPG and polyamines, that correlate with red cell characteristics and clinical severity.
Hence, our data show that untargeted metabolomics in DBS is instrumental in investigating phenotypic heterogeneity and it provides promising leads for studying pathophysiological mechanisms in HS.
Keyword(s): Hemolytic anemia, Hereditary spherocytosis, Red blood cell


