
Contributions
Abstract: S270
Type: Oral Presentation
Session title: Changing the scene on congenital anemias
Background
Pyruvate kinase (PK) deficiency is a rare hereditary disease caused by reduced red blood cell PK (PKR) enzyme activity leading to defective glycolysis. The resulting lifelong hemolytic anemia is associated with serious complications, regardless of transfusion status. Management is limited to supportive therapies. Mitapivat (AG-348) is an investigational, first-in-class, oral, allosteric activator of PKR, which was shown to improve hemolysis for up to 42 months in a phase (Ph.) 2 study of patients (pts) with PK deficiency.
Aims
The ACTIVATE study (NCT03548220) evaluated the efficacy and safety of mitapivat in adult pts with PK deficiency who were not regularly transfused.
Methods
ACTIVATE was a Ph. 3, randomized, double-blind, placebo (PBO)-controlled study. A 12-week (wk) dose escalation (5, 20, 50 mg twice daily) period (Part 1) was followed by a 12-wk fixed-dose period (Part 2). Primary endpoint was hemoglobin (Hb) response, defined as ≥1.5 g/dL increase in Hb from baseline (BL), sustained at ≥2 scheduled assessments at Wks 16, 20, or 24 during Part 2. Secondary endpoints were prespecified and tested in a hierarchical order at a 2-sided α=0.05 as follows: change from BL in Hb concentration, indirect bilirubin, reticulocyte percentage (%), and Hochberg testing for change from BL in lactate dehydrogenase (LDH), haptoglobin, and PK deficiency diary (PKDD) and PK deficiency impact assessment (PKDIA). The PKDD and PKDIA are disease-specific patient-reported outcomes (PROs) in which a higher score indicates more severe disease impact.
Results
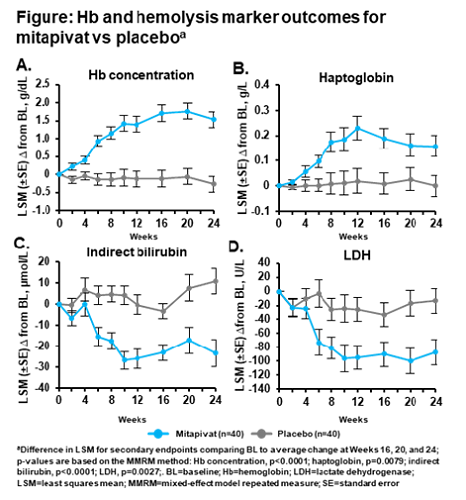
Eighty pts were randomized (mitapivat n=40; PBO n=40). The population was balanced between the mitapivat and PBO arms: mean age 36.0 vs 37.2 years, 40% vs 40% male, 70% vs 80% white, mean BL Hb 8.6 vs 8.5 g/dL. The primary endpoint was met, with 16 mitapivat pts (40%) achieving a sustained Hb response vs 0 PBO pts (p<0.0001). Secondary endpoints were met, including significant improvements with mitapivat compared with PBO in BL Hb, hemolysis markers, and PROs: average change from BL (difference in least squares mean [95% CI]) in Hb concentration, 1.8 g/dL (1.2, 2.4; p<0.0001), indirect bilirubin, –26.26 µmol/L (–37.82, –14.70; p<0.0001), reticulocyte %, –0.1011 (–0.1391, –0.0632; p<0.0001), LDH, –70.81 U/L (–115.88, –25.74; p=0.0027), haptoglobin 0.158 g/L (0.043, 0.273; p=0.0079), and PKDD –3.11 (–5.80, –0.41; p=0.0247) and PKDIA –3.25 (–6.39, –0.12; p=0.0421). The Hb and hemolysis marker improvements in the mitapivat group were rapid and maintained over time (Figure). Treatment-emergent adverse events (TEAEs) occurred in 35 pts in each arm. TEAEs grade ≥3 occurred in 10 (25.0%) mitapivat vs 5 (12.8%) PBO pts. The most common TEAEs with mitapivat were nausea and headache, which were less frequent for mitapivat vs PBO (n=7; 17.5% vs n=9; 23.1% and n=6; 15.0% vs n=13; 33.3%, respectively). The most common TEAEs grade ≥3 with mitapivat were hypertriglyceridemia and hypertension (both n=2; 5.0%). Transaminase increases any grade/grade ≥3 occurred in 1 (2.5%)/0 (0%) mitapivat pt vs 6 (15.4%)/2 (5.1%) PBO pts. No TEAEs led to discontinuation.
Conclusion
ACTIVATE is the first PBO-controlled study in PK deficiency. Primary and secondary endpoints were met, indicating clinically meaningful benefit for pts treated with mitapivat. No new safety signals were identified. Mitapivat has the potential to be the first disease-modifying drug therapy approved for PK deficiency.
Keyword(s): AG-348, Anemia, Hemolysis, Pyruvate kinase deficiency
Abstract: S270
Type: Oral Presentation
Session title: Changing the scene on congenital anemias
Background
Pyruvate kinase (PK) deficiency is a rare hereditary disease caused by reduced red blood cell PK (PKR) enzyme activity leading to defective glycolysis. The resulting lifelong hemolytic anemia is associated with serious complications, regardless of transfusion status. Management is limited to supportive therapies. Mitapivat (AG-348) is an investigational, first-in-class, oral, allosteric activator of PKR, which was shown to improve hemolysis for up to 42 months in a phase (Ph.) 2 study of patients (pts) with PK deficiency.
Aims
The ACTIVATE study (NCT03548220) evaluated the efficacy and safety of mitapivat in adult pts with PK deficiency who were not regularly transfused.
Methods
ACTIVATE was a Ph. 3, randomized, double-blind, placebo (PBO)-controlled study. A 12-week (wk) dose escalation (5, 20, 50 mg twice daily) period (Part 1) was followed by a 12-wk fixed-dose period (Part 2). Primary endpoint was hemoglobin (Hb) response, defined as ≥1.5 g/dL increase in Hb from baseline (BL), sustained at ≥2 scheduled assessments at Wks 16, 20, or 24 during Part 2. Secondary endpoints were prespecified and tested in a hierarchical order at a 2-sided α=0.05 as follows: change from BL in Hb concentration, indirect bilirubin, reticulocyte percentage (%), and Hochberg testing for change from BL in lactate dehydrogenase (LDH), haptoglobin, and PK deficiency diary (PKDD) and PK deficiency impact assessment (PKDIA). The PKDD and PKDIA are disease-specific patient-reported outcomes (PROs) in which a higher score indicates more severe disease impact.
Results
Eighty pts were randomized (mitapivat n=40; PBO n=40). The population was balanced between the mitapivat and PBO arms: mean age 36.0 vs 37.2 years, 40% vs 40% male, 70% vs 80% white, mean BL Hb 8.6 vs 8.5 g/dL. The primary endpoint was met, with 16 mitapivat pts (40%) achieving a sustained Hb response vs 0 PBO pts (p<0.0001). Secondary endpoints were met, including significant improvements with mitapivat compared with PBO in BL Hb, hemolysis markers, and PROs: average change from BL (difference in least squares mean [95% CI]) in Hb concentration, 1.8 g/dL (1.2, 2.4; p<0.0001), indirect bilirubin, –26.26 µmol/L (–37.82, –14.70; p<0.0001), reticulocyte %, –0.1011 (–0.1391, –0.0632; p<0.0001), LDH, –70.81 U/L (–115.88, –25.74; p=0.0027), haptoglobin 0.158 g/L (0.043, 0.273; p=0.0079), and PKDD –3.11 (–5.80, –0.41; p=0.0247) and PKDIA –3.25 (–6.39, –0.12; p=0.0421). The Hb and hemolysis marker improvements in the mitapivat group were rapid and maintained over time (Figure). Treatment-emergent adverse events (TEAEs) occurred in 35 pts in each arm. TEAEs grade ≥3 occurred in 10 (25.0%) mitapivat vs 5 (12.8%) PBO pts. The most common TEAEs with mitapivat were nausea and headache, which were less frequent for mitapivat vs PBO (n=7; 17.5% vs n=9; 23.1% and n=6; 15.0% vs n=13; 33.3%, respectively). The most common TEAEs grade ≥3 with mitapivat were hypertriglyceridemia and hypertension (both n=2; 5.0%). Transaminase increases any grade/grade ≥3 occurred in 1 (2.5%)/0 (0%) mitapivat pt vs 6 (15.4%)/2 (5.1%) PBO pts. No TEAEs led to discontinuation.

Conclusion
ACTIVATE is the first PBO-controlled study in PK deficiency. Primary and secondary endpoints were met, indicating clinically meaningful benefit for pts treated with mitapivat. No new safety signals were identified. Mitapivat has the potential to be the first disease-modifying drug therapy approved for PK deficiency.
Keyword(s): AG-348, Anemia, Hemolysis, Pyruvate kinase deficiency


