
Contributions
Abstract: S255
Type: Oral Presentation
Session title: Cellular immunotherapy and gene therapy - Clinical
Background:
Introduction -Pola -BR (Polatuzumab -bendamustin- rituximab) and chimeric antigen receptor (CAR)-T cells provide superior outcome compared to conventional chemotherapy in patients with relapsed/refractory diffuse large B cell lymphoma (R/R DLBCL). However, how to sequence these strategies remains controversial.
Methods:
The study included R/R DLBCL patients, treated between 01/2019-08/2020 with commercial CAR-T or Pola/Pola BR after failing ≥2 lines of treatment. Propensity score analysis, matching patients based on age, lymphoma category (de-novo/ transformed), cell of origin, number of prior therapy lines, ECOG performance status and LDH level, was performed. Response rate, progression free survival (PFS) and overall survival (OS) were analyzed.
Results:
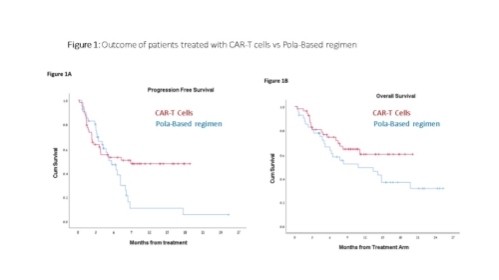
98 patients, treated with CAR-T (n=49; 35 with Tisagenlecleucel, and 14 with Axicabtagene ciloleucel) or Pola-based regimen (n=49) were included (patient characteristics are presented in Table 1). Median time from progressive disease to CAR-T infusion was 52 days and mostly immediate for Pola/Pola-BR. Non-relapse mortality was 0 in the CAR-T cohort vs 6% (3/49) in the Pola arm. The overall and complete response rates were 73% and 53% for the CAR-T cohort vs 63% and 20% in the Pola arm. Within a median follow-up period of 9.6 (range, 1-19.1) and 7.7 (range, 0.7-26) months for CAR-T and Pola patients, respectively, median PFS were 8.9 month (95% CI n/a) vs. 5.6 months (95% CI 3.7-7.6) ( p=0.08) and median OS was not reached vs. 10.8 (2.2-19.4) months, (p=0.12), respectively( Figures 1A, 1B).
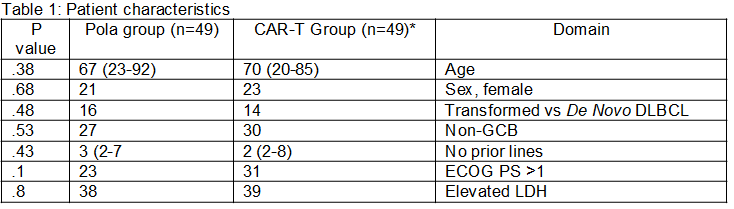
CAR-T- Chimeric Antigen Receptor- T cell ;DLBCL- diffuse large cell B cell lymphoma; ECOG PS- Eastern Cooperative Oncology Group performance status; LDH- ; lactic dehydrogenase; No- number Pola-polatuzumab vedotin Axicabtagene ciloleucel, N=14; Tisagenlecleucel, N=35*
Conclusion:
Conclusions - In the lack of prospective randomized trials evaluating CAR-T s vs chemo-immunotherapy, a propensity score, comparing CAR-T with Pola-based regimen was performed, demonstrating a tendency for prolonged PFS and OS in R/R DLBCL patients treated with CAR-T.
Keyword(s):
Abstract: S255
Type: Oral Presentation
Session title: Cellular immunotherapy and gene therapy - Clinical
Background:
Introduction -Pola -BR (Polatuzumab -bendamustin- rituximab) and chimeric antigen receptor (CAR)-T cells provide superior outcome compared to conventional chemotherapy in patients with relapsed/refractory diffuse large B cell lymphoma (R/R DLBCL). However, how to sequence these strategies remains controversial.
Methods:
The study included R/R DLBCL patients, treated between 01/2019-08/2020 with commercial CAR-T or Pola/Pola BR after failing ≥2 lines of treatment. Propensity score analysis, matching patients based on age, lymphoma category (de-novo/ transformed), cell of origin, number of prior therapy lines, ECOG performance status and LDH level, was performed. Response rate, progression free survival (PFS) and overall survival (OS) were analyzed.
Results:
98 patients, treated with CAR-T (n=49; 35 with Tisagenlecleucel, and 14 with Axicabtagene ciloleucel) or Pola-based regimen (n=49) were included (patient characteristics are presented in Table 1). Median time from progressive disease to CAR-T infusion was 52 days and mostly immediate for Pola/Pola-BR. Non-relapse mortality was 0 in the CAR-T cohort vs 6% (3/49) in the Pola arm. The overall and complete response rates were 73% and 53% for the CAR-T cohort vs 63% and 20% in the Pola arm. Within a median follow-up period of 9.6 (range, 1-19.1) and 7.7 (range, 0.7-26) months for CAR-T and Pola patients, respectively, median PFS were 8.9 month (95% CI n/a) vs. 5.6 months (95% CI 3.7-7.6) ( p=0.08) and median OS was not reached vs. 10.8 (2.2-19.4) months, (p=0.12), respectively( Figures 1A, 1B).

CAR-T- Chimeric Antigen Receptor- T cell ;DLBCL- diffuse large cell B cell lymphoma; ECOG PS- Eastern Cooperative Oncology Group performance status; LDH- ; lactic dehydrogenase; No- number Pola-polatuzumab vedotin Axicabtagene ciloleucel, N=14; Tisagenlecleucel, N=35*

Conclusion:
Conclusions - In the lack of prospective randomized trials evaluating CAR-T s vs chemo-immunotherapy, a propensity score, comparing CAR-T with Pola-based regimen was performed, demonstrating a tendency for prolonged PFS and OS in R/R DLBCL patients treated with CAR-T.
Keyword(s):


