
Contributions
Abstract: S244
Type: Oral Presentation
Session title: Stem cell transplantation - Clinical
Background
Management of the patients with peripheral T-cell lymphoma-not otherwise specified (PTCL-NOS) and angioimmunoblastic T-cell lymphoma (AITL) who failed first-line therapy is a difficult challenge. Salvage chemotherapy followed by autologous hematopoietic cell transplantation (auto-HCT) or allogeneic HCT (allo-HCT) represents the treatment of choice for appropriate candidates. However, there is no consensus on which type of HCT should be applied for such patients.
Aims
To evaluate the impact of auto- or allo-HCT on outcome in PTCL-NOS and AITL failing first-line therapy.
Methods
We performed a retrospective analysis using registry data from the Japan Society for Hematopoietic Cell Transplantation. Adult patients who underwent a first HCT after 2, 3, or 4 lines of therapy ––which means first-line therapy failed for some reason–– between 2006 and 2018 were included.
Results
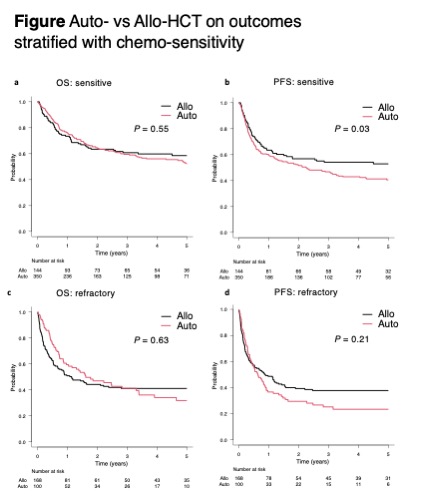
A total of 762 patients with PTCL-NOS (n = 449) and AITL (n = 313) undergoing auto-HCT (n = 450) or allo-HCT (n = 312) as their first HCT were analyzed. Among them, 494 were chemo-sensitive (172 were first complete-remission, 112 were first partial-remission, and 210 were relapsed-sensitive) and 268 were chemo-refractory (142 were primary-refractory and 126 were relapsed-refractory). The allo-HCT recipients were more likely younger (median ages, 53 vs 58 years; P < 0.001), received more than 2 lines of therapy prior to HCT (63% vs 33%; P < 0.001), with higher HCT-comorbidity index (P < 0.001) and with worse performance status (P < 0.001) compared with the auto-HCT recipients. The time from the diagnosis to auto-HCT and allo-HCT were 11 months (range = 2-214) and 11 months (range = 2-154) (P = 0.64), respectively. The median follow-up duration for survivors of auto-HCT and allo-HCT were 36 months (range = 1-150) and 54 months (range = 3-160), respectively. For chemo-sensitive patients, the 4-year overall survival (OS) of auto-HCT and allo-HCT recipients were 56% (95% CI: 49.9-61.7) and 59.8% (95% CI: 50.8-67.8), and the 4-year progression-free survival (PFS) of both groups were 43% (95% CI: 37.2-48.6) and 54.2% (45.2-62.2), respectively (Figure). For chemo-refractory patients, the 4-year OS of auto-HCT and allo-HCT were 34.1% (95% CI: 23.5-44.9) and 41.1% (95% CI: 33.4-48.7), and 4-year PFS of both groups were 23.4% (95% CI: 14.9-33) and 37.8% (95% CI: 30.2-45.2), respectively (Figure). The 2-year non-relapse mortality of auto-HCT and allo-HCT recipients with chemo-sensitive disease were 9.7% (95% CI: 6.7-13.3) and 23.3% (95% CI: 16.6-30.7), while those with chemo-refractory disease were 9.7% (95% CI: 4.7-16.8) and 36.3% (95% CI: 29-43.7), respectively. In the multivariable cox analysis, allo-HCT was a significantly better prognostic factor for PFS in chemo-sensitive patients (hazard ratio [HR], 0.68; 95% CI: 0.5-0.92; P = 0.01), and marginally better for PFS in.chemo-refractory patients (HR, 0.73; 95% CI: 0.52-1.01; P = 0.06). The subgroup analyses suggested that allo-HCT tended to be associated with better OS for relapsed-sensitive disease (HR 0.61; 95% CI: 0.36-1.04; P = 0.07) among chemo-sensitive patients, and primary-refractory disease (HR 0.51; 95% CI: 0.29-0.89; P = 0.02) among chemo-refractory patients.
Conclusion
These data showed allo-HCT for PTCL-NOS and AITL failing first-line therapy can result in comparable survival outcomes despite being with more unfavorable factors compared with auto-HCT. Patients with relapsed-sensitive and primary-refractory disease may be a good candidate for allo-HCT if suitable donor is available.
Keyword(s): T cell lymphoma, Transplant
Abstract: S244
Type: Oral Presentation
Session title: Stem cell transplantation - Clinical
Background
Management of the patients with peripheral T-cell lymphoma-not otherwise specified (PTCL-NOS) and angioimmunoblastic T-cell lymphoma (AITL) who failed first-line therapy is a difficult challenge. Salvage chemotherapy followed by autologous hematopoietic cell transplantation (auto-HCT) or allogeneic HCT (allo-HCT) represents the treatment of choice for appropriate candidates. However, there is no consensus on which type of HCT should be applied for such patients.
Aims
To evaluate the impact of auto- or allo-HCT on outcome in PTCL-NOS and AITL failing first-line therapy.
Methods
We performed a retrospective analysis using registry data from the Japan Society for Hematopoietic Cell Transplantation. Adult patients who underwent a first HCT after 2, 3, or 4 lines of therapy ––which means first-line therapy failed for some reason–– between 2006 and 2018 were included.
Results
A total of 762 patients with PTCL-NOS (n = 449) and AITL (n = 313) undergoing auto-HCT (n = 450) or allo-HCT (n = 312) as their first HCT were analyzed. Among them, 494 were chemo-sensitive (172 were first complete-remission, 112 were first partial-remission, and 210 were relapsed-sensitive) and 268 were chemo-refractory (142 were primary-refractory and 126 were relapsed-refractory). The allo-HCT recipients were more likely younger (median ages, 53 vs 58 years; P < 0.001), received more than 2 lines of therapy prior to HCT (63% vs 33%; P < 0.001), with higher HCT-comorbidity index (P < 0.001) and with worse performance status (P < 0.001) compared with the auto-HCT recipients. The time from the diagnosis to auto-HCT and allo-HCT were 11 months (range = 2-214) and 11 months (range = 2-154) (P = 0.64), respectively. The median follow-up duration for survivors of auto-HCT and allo-HCT were 36 months (range = 1-150) and 54 months (range = 3-160), respectively. For chemo-sensitive patients, the 4-year overall survival (OS) of auto-HCT and allo-HCT recipients were 56% (95% CI: 49.9-61.7) and 59.8% (95% CI: 50.8-67.8), and the 4-year progression-free survival (PFS) of both groups were 43% (95% CI: 37.2-48.6) and 54.2% (45.2-62.2), respectively (Figure). For chemo-refractory patients, the 4-year OS of auto-HCT and allo-HCT were 34.1% (95% CI: 23.5-44.9) and 41.1% (95% CI: 33.4-48.7), and 4-year PFS of both groups were 23.4% (95% CI: 14.9-33) and 37.8% (95% CI: 30.2-45.2), respectively (Figure). The 2-year non-relapse mortality of auto-HCT and allo-HCT recipients with chemo-sensitive disease were 9.7% (95% CI: 6.7-13.3) and 23.3% (95% CI: 16.6-30.7), while those with chemo-refractory disease were 9.7% (95% CI: 4.7-16.8) and 36.3% (95% CI: 29-43.7), respectively. In the multivariable cox analysis, allo-HCT was a significantly better prognostic factor for PFS in chemo-sensitive patients (hazard ratio [HR], 0.68; 95% CI: 0.5-0.92; P = 0.01), and marginally better for PFS in.chemo-refractory patients (HR, 0.73; 95% CI: 0.52-1.01; P = 0.06). The subgroup analyses suggested that allo-HCT tended to be associated with better OS for relapsed-sensitive disease (HR 0.61; 95% CI: 0.36-1.04; P = 0.07) among chemo-sensitive patients, and primary-refractory disease (HR 0.51; 95% CI: 0.29-0.89; P = 0.02) among chemo-refractory patients.

Conclusion
These data showed allo-HCT for PTCL-NOS and AITL failing first-line therapy can result in comparable survival outcomes despite being with more unfavorable factors compared with auto-HCT. Patients with relapsed-sensitive and primary-refractory disease may be a good candidate for allo-HCT if suitable donor is available.
Keyword(s): T cell lymphoma, Transplant


