
Contributions
Abstract: S239
Type: Oral Presentation
Session title: Stem cell transplantation - GvHD
Background
Belumosudil (KD025) is a novel oral selective rho-associated coiled-coil kinase 2 (ROCK2) inhibitor specifically designed for the treatment of cGVHD, an immune-mediated inflammatory and fibrotic disorder.
Aims
In a previous dose-finding study (KD025-208, N=54), two-thirds of patients achieved a partial or complete response with belumosudil. Herein, we report 12 month updated results from the pivotal randomized phase 2 trial (ROCKstar [KD025-213], N=132).
Methods
We randomly assigned cGVHD patients (pts) after 2-5 prior lines of therapy (LOT) to belumosudil 200mg QD (n=66), or belumosudil 200mg BID (n=66), across 28 sites, stratified according to cGVHD severity and prior ibrutinib use. The primary endpoint was the overall response rate (ORR) per 2014 NIH response criteria, assessed by investigators. Additional endpoints include duration of response, Failure-Free Survival (FFS), improvement in symptoms based on change in Lee symptom score (LSS) and corticosteroid (CS) dose reductions or discontinuations. Treatment was until clinically significant progression or unacceptable toxicity.
Results
Pooled baseline characteristics of the two arms revealed a median age of 56 (21-77); median prior LOT 3; median time from cGVHD diagnosis 29 mos (2-162); 67% with NIH defined severe cGVHD; 34% with prior ibrutinib use; 52% with ≥ 4 organ involvement; and 72% refractory to prior line of therapy
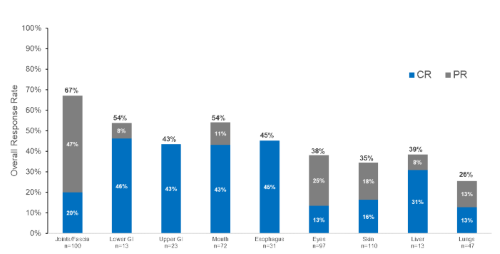
The ORR [95% CI] was 73% [60, 83] with belumosudil QD and 77% [65, 87] with belumosudil BID. Responses were consistent across key subgroups and complete responses were observed in all organ systems as shown in Figure 1. Furthermore, in patients with previous ibrutinib or ruxolitinib use, ORRs were 74% [59, 86] and 68% [51, 83], respectively. The median DOR was 50 weeks. FFS was 76% (68%>83%) at 6 months. Sixty percent pts reported a clinically meaningful improvement (≥7 point reduction) in LSS score. Corticosteroids were discontinued in 21% of patients.
Belumosudil was well tolerated, and AEs were consistent with those expected in this population. AEs (QD, BID) in >20% pts were fatigue (38%), diarrhea (33%), nausea (31%), cough (28%), upper respiratory infections (27%), dyspnea (25%), headache (24%), peripheral edema (23%), vomiting (21%) and muscle spasms (20%). At least 1 serious AE occurred in 38% of patients. Fourteen patients died during the study; 8 due to AEs (2 possibly related to belumosudil) and 6 during long term follow-up (>28 days after last dose). There was a report of 1 case of Epstein-Barr viremia, and 1 report of cytomegalovirus reactivation.
Conclusion
Treatment with belumosudil was well tolerated at both doses and resulted in high ORRs across key subgroups, meeting the primary end point of this pivotal randomized trial in cGVHD. Responses were durable and clinically meaningful, irrespective of patient and cGVHD characteristics and organ involvement.
Keyword(s):
Abstract: S239
Type: Oral Presentation
Session title: Stem cell transplantation - GvHD
Background
Belumosudil (KD025) is a novel oral selective rho-associated coiled-coil kinase 2 (ROCK2) inhibitor specifically designed for the treatment of cGVHD, an immune-mediated inflammatory and fibrotic disorder.
Aims
In a previous dose-finding study (KD025-208, N=54), two-thirds of patients achieved a partial or complete response with belumosudil. Herein, we report 12 month updated results from the pivotal randomized phase 2 trial (ROCKstar [KD025-213], N=132).
Methods
We randomly assigned cGVHD patients (pts) after 2-5 prior lines of therapy (LOT) to belumosudil 200mg QD (n=66), or belumosudil 200mg BID (n=66), across 28 sites, stratified according to cGVHD severity and prior ibrutinib use. The primary endpoint was the overall response rate (ORR) per 2014 NIH response criteria, assessed by investigators. Additional endpoints include duration of response, Failure-Free Survival (FFS), improvement in symptoms based on change in Lee symptom score (LSS) and corticosteroid (CS) dose reductions or discontinuations. Treatment was until clinically significant progression or unacceptable toxicity.
Results
Pooled baseline characteristics of the two arms revealed a median age of 56 (21-77); median prior LOT 3; median time from cGVHD diagnosis 29 mos (2-162); 67% with NIH defined severe cGVHD; 34% with prior ibrutinib use; 52% with ≥ 4 organ involvement; and 72% refractory to prior line of therapy
The ORR [95% CI] was 73% [60, 83] with belumosudil QD and 77% [65, 87] with belumosudil BID. Responses were consistent across key subgroups and complete responses were observed in all organ systems as shown in Figure 1. Furthermore, in patients with previous ibrutinib or ruxolitinib use, ORRs were 74% [59, 86] and 68% [51, 83], respectively. The median DOR was 50 weeks. FFS was 76% (68%>83%) at 6 months. Sixty percent pts reported a clinically meaningful improvement (≥7 point reduction) in LSS score. Corticosteroids were discontinued in 21% of patients.
Belumosudil was well tolerated, and AEs were consistent with those expected in this population. AEs (QD, BID) in >20% pts were fatigue (38%), diarrhea (33%), nausea (31%), cough (28%), upper respiratory infections (27%), dyspnea (25%), headache (24%), peripheral edema (23%), vomiting (21%) and muscle spasms (20%). At least 1 serious AE occurred in 38% of patients. Fourteen patients died during the study; 8 due to AEs (2 possibly related to belumosudil) and 6 during long term follow-up (>28 days after last dose). There was a report of 1 case of Epstein-Barr viremia, and 1 report of cytomegalovirus reactivation.

Conclusion
Treatment with belumosudil was well tolerated at both doses and resulted in high ORRs across key subgroups, meeting the primary end point of this pivotal randomized trial in cGVHD. Responses were durable and clinically meaningful, irrespective of patient and cGVHD characteristics and organ involvement.
Keyword(s):


