
Contributions
Abstract: S238
Type: Oral Presentation
Session title: Stem cell transplantation - GvHD
Background
CD6 is a co-stimulatory receptor predominantly expressed on T cells. CD6high CD4+ T cells were recently shown to drive Th1/Th17 immune responses in inflammatory bowel disease and may have a similar role in acute graft-versus-host disease (aGHVD). The CD6 ligand, activated leukocyte cell adhesion molecule (ALCAM), is expressed on antigen presenting cells, as well as epithelial and endothelial cells of aGVHD target organs (e.g. skin, GI tract). Previous studies in patients receiving allogeneic hematopoietic cell transplants showed that ex vivo depletion of donor CD6+ T cells lowered the incidence of aGVHD, providing a rationale for therapeutically targeting CD6 in aGVHD. Itolizumab is a humanized IgG1 monoclonal antibody that binds CD6 and blocks interaction with ALCAM to inhibit T cell activity and trafficking that is being evaluated as treatment for aGVHD.
Aims
Here we present interim study results from the Primary Cohort of EQUATE, an ongoing US-based Phase 1b/2 study of itolizumab in combination with steroids for newly diagnosed aGVHD.
Methods
Phase 1b is an open-label, dose-escalation study evaluating doses from 0.4 to 2.4 mg/kg (IV Q2 weeks through Day 57). The Primary Cohort enrolled patients with Grade III-IV aGVHD that received itolizumab within 72 hours of first steroid dose. The Expansion Cohort also includes subjects with Grade II aGVHD and an Ann Arbor [AA] Score of 2 or 3 and who received itolizumab within 7 days of first steroid dose.
Results
A total of 10 subjects in the Primary Cohort have completed treatment through Day 85: 0.4 mg/kg (n=4), 0.8 mg/kg (n=3), and 1.6 mg/kg (n=3). Baseline characteristics were mean age of 48, 90% male, 90% white, 80% with peripheral blood graft source, 80% with HLA matched donor, mean time to GVHD onset of 43 days, and 100% with GI involvement. Mean MAGIC algorithm probability (MAP) was 0.468, and 70% had an AA score of 3. One subject in the 0.4 mg/kg cohort received only one itolizumab dose, whereas all other subjects received 2-5 doses.
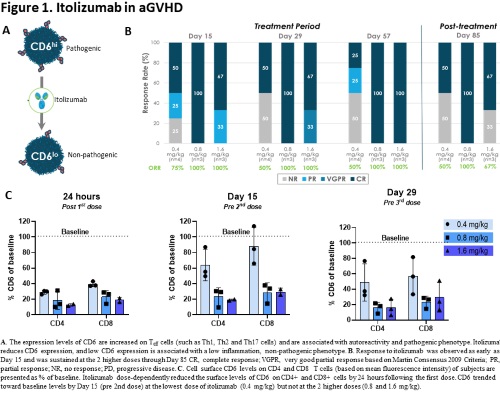
Across all doses, the overall response rate (ORR) was 80% at Day 29, with 70% of subjects experiencing a complete response (CR) and 10% experiencing a very good partial response (VGPR). ORR was 70% at Day 85 with 100% of responders experiencing CR (Fig 1B). Response was consistent through Day 169. MAP scores also decreased through Day 85 with a median % decrease from baseline of 18%. At Day 85, median % steroid dose reduction was 89%. Itolizumab dose-dependently decreased CD6 levels on T cells within 24 hours of first dose, which was maintained throughout the treatment period, with a more pronounced effect in the 0.8 and 1.6 mg/kg (Fig 1C).
Safety profile is consistent with that of itolizumab observed to date across other trials and with adverse events (AEs) common in this patient population. All subjects experienced at least 1 AE. Most AEs were mild to moderate in severity. One mild infusion reaction AE was noted. Serious AEs were noted in 5 subjects, including recurrent gut GVHD (n=1), sepsis (n=2; 1 was considered a DLT) and fever (n=1), COVID-19 (n=1) and nocardiosis (n=1), physical deconditioning (n=1), and atrial flutter (n=1). There was one death reported due to an SAE of intestinal infarction deemed not related to study drug. Another death occurred >100 days post dose due to progressive aGVHD and was also not related to study drug.
Conclusion
In summary, the safety and efficacy observed to date and benefit-risk profile support continued study and evaluation in future randomized controlled trials.
Keyword(s): Acute graft-versus-host disease, CD4+ T cells, CD8 T cells, Clinical trial
Abstract: S238
Type: Oral Presentation
Session title: Stem cell transplantation - GvHD
Background
CD6 is a co-stimulatory receptor predominantly expressed on T cells. CD6high CD4+ T cells were recently shown to drive Th1/Th17 immune responses in inflammatory bowel disease and may have a similar role in acute graft-versus-host disease (aGHVD). The CD6 ligand, activated leukocyte cell adhesion molecule (ALCAM), is expressed on antigen presenting cells, as well as epithelial and endothelial cells of aGVHD target organs (e.g. skin, GI tract). Previous studies in patients receiving allogeneic hematopoietic cell transplants showed that ex vivo depletion of donor CD6+ T cells lowered the incidence of aGVHD, providing a rationale for therapeutically targeting CD6 in aGVHD. Itolizumab is a humanized IgG1 monoclonal antibody that binds CD6 and blocks interaction with ALCAM to inhibit T cell activity and trafficking that is being evaluated as treatment for aGVHD.
Aims
Here we present interim study results from the Primary Cohort of EQUATE, an ongoing US-based Phase 1b/2 study of itolizumab in combination with steroids for newly diagnosed aGVHD.
Methods
Phase 1b is an open-label, dose-escalation study evaluating doses from 0.4 to 2.4 mg/kg (IV Q2 weeks through Day 57). The Primary Cohort enrolled patients with Grade III-IV aGVHD that received itolizumab within 72 hours of first steroid dose. The Expansion Cohort also includes subjects with Grade II aGVHD and an Ann Arbor [AA] Score of 2 or 3 and who received itolizumab within 7 days of first steroid dose.
Results
A total of 10 subjects in the Primary Cohort have completed treatment through Day 85: 0.4 mg/kg (n=4), 0.8 mg/kg (n=3), and 1.6 mg/kg (n=3). Baseline characteristics were mean age of 48, 90% male, 90% white, 80% with peripheral blood graft source, 80% with HLA matched donor, mean time to GVHD onset of 43 days, and 100% with GI involvement. Mean MAGIC algorithm probability (MAP) was 0.468, and 70% had an AA score of 3. One subject in the 0.4 mg/kg cohort received only one itolizumab dose, whereas all other subjects received 2-5 doses.
Across all doses, the overall response rate (ORR) was 80% at Day 29, with 70% of subjects experiencing a complete response (CR) and 10% experiencing a very good partial response (VGPR). ORR was 70% at Day 85 with 100% of responders experiencing CR (Fig 1B). Response was consistent through Day 169. MAP scores also decreased through Day 85 with a median % decrease from baseline of 18%. At Day 85, median % steroid dose reduction was 89%. Itolizumab dose-dependently decreased CD6 levels on T cells within 24 hours of first dose, which was maintained throughout the treatment period, with a more pronounced effect in the 0.8 and 1.6 mg/kg (Fig 1C).
Safety profile is consistent with that of itolizumab observed to date across other trials and with adverse events (AEs) common in this patient population. All subjects experienced at least 1 AE. Most AEs were mild to moderate in severity. One mild infusion reaction AE was noted. Serious AEs were noted in 5 subjects, including recurrent gut GVHD (n=1), sepsis (n=2; 1 was considered a DLT) and fever (n=1), COVID-19 (n=1) and nocardiosis (n=1), physical deconditioning (n=1), and atrial flutter (n=1). There was one death reported due to an SAE of intestinal infarction deemed not related to study drug. Another death occurred >100 days post dose due to progressive aGVHD and was also not related to study drug.

Conclusion
In summary, the safety and efficacy observed to date and benefit-risk profile support continued study and evaluation in future randomized controlled trials.
Keyword(s): Acute graft-versus-host disease, CD4+ T cells, CD8 T cells, Clinical trial


