
Contributions
Abstract: S237
Type: Oral Presentation
Session title: Stem cell transplantation - GvHD
Background
Sclerotic chronic graft-versus-host disease (scGVHD) represents a distinctive phenotypic variant of GVHD that is characterized by dermal fibrosis, often resulting in severe morbidity. scGVHD is associated with high rates of steroid refractoriness, and therapeutic options in this setting remain limited. Aberrant hedgehog signaling is prominent in scGVHD, leading to the activation of dermal fibroblasts. Targeted inhibition of the pathway ameliorates scGVHD features in experimental models.
Aims
This investigator-led study (NCT03415867) evaluated the safety profile and preliminary efficacy of glasdegib, an oral antagonist of the hedgehog signalling transducer Smoothened (SMO), in adult patients (pts) with active scGVHD after ≥2 lines of prior therapy.
Methods
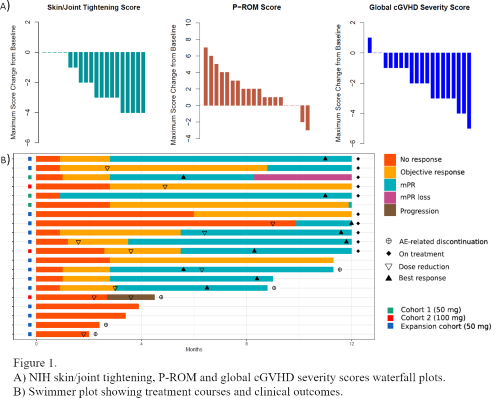
The trial followed a standard 3+3 escalation dose-finding design. Glasdegib was administered once daily (OD) in continuous 28-day cycles. Treatment could be continued beyond Cycle 12 in pts obtaining objective clinical benefit. The primary endpoints of the study were dose-limiting toxicity (DLT) and maximum tolerated dose (MTD). Secondary endpoints included safety, efficacy, PK/PD and immune correlative studies. Responses were assessed per 2014 NIH cGVHD criteria. Additionally, a modified skin/joint partial response category (mPR) was defined as a NIH cGVHD global severity score decrease ≥2 points in the presence of any significant skin/joint scoring improvement (≥1 point for body surface area, skin features or joints/fascia scores; ≥2 points for skin/joint tightening or P-ROM scores). Data cut-off was at the end of Cycle 12.
Results
Twenty pts have been included in the primary analysis. An additional non-DLT-evaluable pt was replaced early after enrolment in Cohort 1 due to a treatment-unrelated cerebrovascular ischemic event. Median age was 47 years (range 27-72). Enrolled pts had failed a median of 4 prior lines of therapy (range 2-10), 50% having received treatment with ruxolitinib. Three pts were included in Cohort 1 (50 mg OD), 3 in Cohort 2 (100 mg OD) and 16 in the expansion cohort (50 mg OD). Two pts in Cohort 2 experienced a DLT (grade [G] 3 muscle cramps), the MTD being thus established at 50 mg OD. Frequent treatment-emergent G≥2 adverse events (AEs) included muscle cramps (85%), alopecia (40%) and dysgeusia (35%). Two pts developed G3 and G4 treatment-related creatine kinase elevations that required interruption of glasdegib but did not result in study discontinuation. Five pts withdrew from the study as a result of treatment-related AEs, and 10 pts required dose adjustments while on treatment. Ten pts remained on the study at the end of Cycle 12.
At the data cut-off, 12 (60%) pts had achieved a NIH global severity score PR, 12 (60%) a joints/fascia response (11 PR, 1 CR) and 3 (15%) a skin PR. An improvement ≥2 points in skin/joint tightening and P-ROM scores was observed in 13 (65%) and 11 (55%) pts, respectively. Overall, 12 (60%) pts obtained a skin/joint mPR, with a median time of 3 months to response and a duration ≥6 months in 7 (35%) pts.
In preliminary analyses, treatment with glasdegib was not associated with significant changes in the distribution of T-cell compartments or TCR diversity.
Conclusion
Low doses of glasdegib demonstrate high rates of clinical responses in heavily pretreated pts with scGVHD, resulting in durable responses in one-third of the pts. Treatment schedule adjustments may be required in order to optimise tolerability.
Keyword(s): Clinical trial, Graft-versus-host disease (GVHD), Stem cell transplant, Targeted therapy
Abstract: S237
Type: Oral Presentation
Session title: Stem cell transplantation - GvHD
Background
Sclerotic chronic graft-versus-host disease (scGVHD) represents a distinctive phenotypic variant of GVHD that is characterized by dermal fibrosis, often resulting in severe morbidity. scGVHD is associated with high rates of steroid refractoriness, and therapeutic options in this setting remain limited. Aberrant hedgehog signaling is prominent in scGVHD, leading to the activation of dermal fibroblasts. Targeted inhibition of the pathway ameliorates scGVHD features in experimental models.
Aims
This investigator-led study (NCT03415867) evaluated the safety profile and preliminary efficacy of glasdegib, an oral antagonist of the hedgehog signalling transducer Smoothened (SMO), in adult patients (pts) with active scGVHD after ≥2 lines of prior therapy.
Methods
The trial followed a standard 3+3 escalation dose-finding design. Glasdegib was administered once daily (OD) in continuous 28-day cycles. Treatment could be continued beyond Cycle 12 in pts obtaining objective clinical benefit. The primary endpoints of the study were dose-limiting toxicity (DLT) and maximum tolerated dose (MTD). Secondary endpoints included safety, efficacy, PK/PD and immune correlative studies. Responses were assessed per 2014 NIH cGVHD criteria. Additionally, a modified skin/joint partial response category (mPR) was defined as a NIH cGVHD global severity score decrease ≥2 points in the presence of any significant skin/joint scoring improvement (≥1 point for body surface area, skin features or joints/fascia scores; ≥2 points for skin/joint tightening or P-ROM scores). Data cut-off was at the end of Cycle 12.
Results
Twenty pts have been included in the primary analysis. An additional non-DLT-evaluable pt was replaced early after enrolment in Cohort 1 due to a treatment-unrelated cerebrovascular ischemic event. Median age was 47 years (range 27-72). Enrolled pts had failed a median of 4 prior lines of therapy (range 2-10), 50% having received treatment with ruxolitinib. Three pts were included in Cohort 1 (50 mg OD), 3 in Cohort 2 (100 mg OD) and 16 in the expansion cohort (50 mg OD). Two pts in Cohort 2 experienced a DLT (grade [G] 3 muscle cramps), the MTD being thus established at 50 mg OD. Frequent treatment-emergent G≥2 adverse events (AEs) included muscle cramps (85%), alopecia (40%) and dysgeusia (35%). Two pts developed G3 and G4 treatment-related creatine kinase elevations that required interruption of glasdegib but did not result in study discontinuation. Five pts withdrew from the study as a result of treatment-related AEs, and 10 pts required dose adjustments while on treatment. Ten pts remained on the study at the end of Cycle 12.
At the data cut-off, 12 (60%) pts had achieved a NIH global severity score PR, 12 (60%) a joints/fascia response (11 PR, 1 CR) and 3 (15%) a skin PR. An improvement ≥2 points in skin/joint tightening and P-ROM scores was observed in 13 (65%) and 11 (55%) pts, respectively. Overall, 12 (60%) pts obtained a skin/joint mPR, with a median time of 3 months to response and a duration ≥6 months in 7 (35%) pts.
In preliminary analyses, treatment with glasdegib was not associated with significant changes in the distribution of T-cell compartments or TCR diversity.

Conclusion
Low doses of glasdegib demonstrate high rates of clinical responses in heavily pretreated pts with scGVHD, resulting in durable responses in one-third of the pts. Treatment schedule adjustments may be required in order to optimise tolerability.
Keyword(s): Clinical trial, Graft-versus-host disease (GVHD), Stem cell transplant, Targeted therapy


